
Atomic Structure and the Periodic table (3.1.1)
Fundamental Particles:
Specification points:
Appreciate that knowledge and understanding of atomic structure has evolved over time.
Protons, neutrons and electrons: relative charge and relative mass.
An atom consists of a nucleus containing protons and neutrons surrounded by electrons.
Most of the mass of the atom is concentrated in the nucleus.
Particle | Relative charge | Relative Mass |
|---|---|---|
Proton | +1 | 1 |
Neutron | 0 | 1 |
Electron | -1 | 1/1840 |
Previous Atomic Models:
Dalton’s model- At the start of the 19th century John Dalton described atoms as solid spheres and said that different spheres made up the different elements.
J.J. Thomson’s model- In 1897 J. J. Thomson concluded from his experiments that an atom must contain even smaller, negatively charged particles — electrons. The ‘solid sphere’ idea of atomic structure had to be changed. The new model was known as the ‘plum pudding model’.

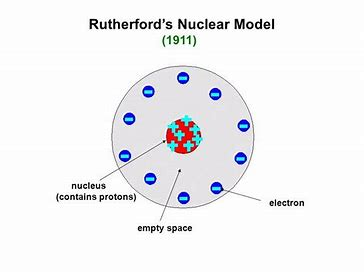
Rutherford’s model- In 1909 Ernest Rutherford and his students Hans Geiger and Ernest Marsden conducted their famous gold foil experiment. They fired alpha particles (which are positively charged) at a very thin sheet of gold. From the plum pudding model, they were expecting most of the alpha particles to be deflected slightly by the positive ‘pudding’ that made up most of an atom. In fact, most of the alpha particles passed straight through the gold atoms and a very small number were deflected backwards — the nuclear model of the atom. In this, there’s a tiny, positively charged nucleus at the centre, surrounded by a ‘cloud’ of negative electrons. Most of the atom is empty space.

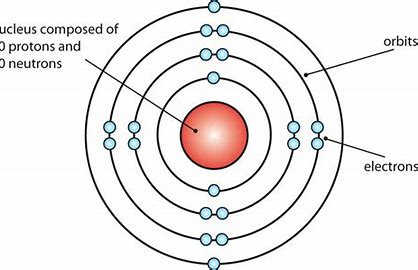
Bohr model- Niels Bohr proposed a new model of the atom with four basic principles: Electrons only exist in fixed orbits (shells) and not anywhere in between.
Each shell has a fixed energy.
When an electron moves between shells electromagnetic radiation is emitted or absorbed. Because the energy of shells is fixed, the radiation will have a fixed frequency.
Mass Number and Isotopes:
Specification Points:
Mass number (A) and atomic (proton) number (Z).
Students should be able to:
determine the number of fundamental particles in atoms and ions using mass number, atomic number and charge
explain the existence of isotopes.
The principles of a simple time of flight (TOF) mass spectrometer, limited to ionisation, acceleration to give all ions constant kinetic energy, ion drift, ion detection, data analysis.
The mass spectrometer gives accurate information about relative isotopic mass and also about the relative abundance of isotopes.
Mass spectrometry can be used to identify elements.
Mass spectrometry can be used to determine relative molecular mass.
Students should be able to:
interpret simple mass spectra of elements
calculate relative atomic mass from isotopic abundance, limited to mononuclear ions.
==Mass number(A) -==This is the total number of protons and neutrons in the nucleus of an atom.
Atomic (proton) number (Z)- This is the number of protons in the nucleus of an atom — it identifies the element. All atoms of the same element have the same number of protons.
For neutral atoms, which have no overall charge, the number of electrons is the same as the number of protons. The number of neutrons is just mass number minus atomic number (top minus bottom in the nuclear symbol).
Ions -Atoms form ions by gaining or losing electrons. Ions have different numbers of protons and electrons — negative ions have more electrons than protons, and positive ions have fewer electrons than protons.
Negative ion: gain of electron(s)
Positive ion: loss of electron(s)
Isotopes of an element are atoms with the same number of protons but different numbers of neutrons. Isotopes have the same configuration of electrons, so they have the same chemical properties.
The relative atomic mass, Ar , is the average mass of an atom of an element on a scale where an atom of carbon-12 is exactly 12. The relative atomic mass of each element is shown in the periodic table.
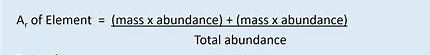
Mass Spectrometry:
The time of flight (TOF) mass spectrometer is an instrument used for measuring the masses of atoms and molecules. It can also be used to measure the relative abundance of different isotopes and to predict the structure of more complex molecules.

Ionisation The sample needs to be ionised before it enters the mass spectrometer. Two ways of doing this are:
Electrospray ionisation, — in this method the sample is dissolved in a solvent and pushed through a small nozzle at high pressure. A high voltage is applied to it, causing each particle to gain an H+ ion. The solvent is then removed, leaving a gas made up of positive ions.
Electron impact ionisation — in this method, the sample is vaporised and an ‘electron gun’ is used to fire high energy electrons at it. This knocks one electron off one each particle, so they become +1 ions.
Acceleration The positive ions are accelerated by an electric field. The electric field gives the same kinetic energy to all the ions. The lighter ions experience a greater acceleration — they’re given as much energy as the heavier ions, but they’re lighter, so they accelerate more.
Ion drift Next, the ions enter a region with no electric field. They drift through it at the same speed as they left the electric field. So, the lighter ions will be drifting at higher speeds.
Detection Because lighter ions travel through the drift region at higher speeds, they reach the detector in less time than heavier ions. The detector detects the current created when the ions hit it and records how long they took to pass through the spectrometer. This data is then used to calculate the mass/charge values needed to produce a mass spectrum.
Electron Arrangement:
Specification Points:
Electron configurations of atoms and ions up to Z = 36 in terms of shells and sub-shells (orbitals) s, p and d.
Ionisation energies.
Students should be able to:
define first ionisation energy
write equations for first and successive ionisation energies
explain how first and successive ionisation energies in Period 3 (Na–Ar) and in Group 2 (Be–Ba) give evidence for electron configuration in sub-shells and in shells.
In the currently accepted model of the atom, electrons have fixed energies. They move around the nucleus in certain regions of the atom called shells or energy levels. Each shell is given a number called the principal quantum number. The further a shell is from the nucleus, the higher its energy and the larger its principal quantum number.
Experiments show that not all the electrons in a shell have exactly the same energy. The atomic model explains this — shells are divided up into sub-shells. Different electron shells have different numbers of sub-shells, which each have a different energy. Sub-shells can be s sub-shells, p sub-shells, d sub-shells or f sub-shells.
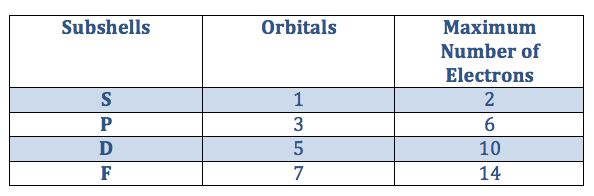
The sub-shells have different numbers of orbitals which can each hold up to 2 electrons. The table on the right shows the number of orbitals in each sub-shell. You can use it to work out the number of electrons that each shell can hold.
The first ionisation energy is the energy needed to remove 1 electron from each atom in 1 mole of gaseous atoms to form 1 mole of gaseous 1+ ions.
Factors affecting ionisation energy:
A high ionisation energy means there’s a high attraction between the electron and the nucleus, so more energy is needed to remove the electron. There are three things that can affect ionisation energy:
==1 Nuclear charge-==the more protons there are in the nucleus, the more positively charged the nucleus is and the stronger the attraction for the electrons.
2 Distance from nucleus- Attraction falls off very rapidly with distance. An electron close to the nucleus will be much more strongly attracted than one further away.
==3 Shielding -==As the number of electrons between the outer electrons and the nucleus increases, the outer electrons feel less attraction to the nucleus. This lessening of the pull of the nucleus thanks to the inner electron shells is called shielding.
You can write equations for this process — here’s the equation for the first ionisation of oxygen:
O(g) +O (g) + e–
Here are a few rather important points about ionisation energies:
You must use the gas state symbol, (g), because ionisation energies are measured for gaseous atoms.
Always refer to 1 mole of atoms, as stated in the definition, rather than to a single atom.
The lower the ionisation energy, the easier it is to form a positive ion.
Ionisation trends down Group 2:
First ionisation energy decreases down Group 2.
This provides evidence that electron shells really exist. If each element going down Group 2 has one more electron shell than the one above, the extra shell will shield the outer electrons from the attraction of the nucleus. Also, the extra shell means that the outer electrons will be further from the nucleus, so the nucleus’s attraction will be reduced. It makes sense that both of these factors make it easier to remove outer electrons, resulting in lower ionisation energies.
Ionisation trends across periods:
The graph below shows the first ionisation energies of the elements in Period 3.
As you move across a period, the general trend is for the ionisation energies to increase — i.e. it gets harder to remove the outer electrons. This can be explained by the fact that the number of protons is increasing, which means a stronger nuclear attraction.
Ionisation energies and shell structure:
If you know the successive ionisation energies of an element you can work out the number of electrons in each shell of the atom and which group the element is in.
Within each shell, successive ionisation energies increase. This is because electrons are being removed from an increasingly positive ion — there’s less repulsion amongst the remaining electrons, so they’re held more strongly by the nucleus. The big jumps in ionisation energy happen when a new shell is broken into — an electron is being removed from a shell closer to the nucleus.
Atomic Structure and the Periodic table (3.1.1)
Fundamental Particles:
Specification points:
Appreciate that knowledge and understanding of atomic structure has evolved over time.
Protons, neutrons and electrons: relative charge and relative mass.
An atom consists of a nucleus containing protons and neutrons surrounded by electrons.
Most of the mass of the atom is concentrated in the nucleus.
Particle | Relative charge | Relative Mass |
|---|---|---|
Proton | +1 | 1 |
Neutron | 0 | 1 |
Electron | -1 | 1/1840 |
Previous Atomic Models:
Dalton’s model- At the start of the 19th century John Dalton described atoms as solid spheres and said that different spheres made up the different elements.

J.J. Thomson’s model- In 1897 J. J. Thomson concluded from his experiments that an atom must contain even smaller, negatively charged particles — electrons. The ‘solid sphere’ idea of atomic structure had to be changed. The new model was known as the ‘plum pudding model’.

Rutherford’s model- In 1909 Ernest Rutherford and his students Hans Geiger and Ernest Marsden conducted their famous gold foil experiment. They fired alpha particles (which are positively charged) at a very thin sheet of gold. From the plum pudding model, they were expecting most of the alpha particles to be deflected slightly by the positive ‘pudding’ that made up most of an atom. In fact, most of the alpha particles passed straight through the gold atoms and a very small number were deflected backwards — the nuclear model of the atom. In this, there’s a tiny, positively charged nucleus at the centre, surrounded by a ‘cloud’ of negative electrons. Most of the atom is empty space.


Bohr model- Niels Bohr proposed a new model of the atom with four basic principles: Electrons only exist in fixed orbits (shells) and not anywhere in between.
Each shell has a fixed energy.
When an electron moves between shells electromagnetic radiation is emitted or absorbed. Because the energy of shells is fixed, the radiation will have a fixed frequency.

Mass Number and Isotopes:
Specification Points:
Mass number (A) and atomic (proton) number (Z).
Students should be able to:
determine the number of fundamental particles in atoms and ions using mass number, atomic number and charge
explain the existence of isotopes.
The principles of a simple time of flight (TOF) mass spectrometer, limited to ionisation, acceleration to give all ions constant kinetic energy, ion drift, ion detection, data analysis.
The mass spectrometer gives accurate information about relative isotopic mass and also about the relative abundance of isotopes.
Mass spectrometry can be used to identify elements.
Mass spectrometry can be used to determine relative molecular mass.
Students should be able to:
interpret simple mass spectra of elements
calculate relative atomic mass from isotopic abundance, limited to mononuclear ions.
==Mass number(A) -==This is the total number of protons and neutrons in the nucleus of an atom.
Atomic (proton) number (Z)- This is the number of protons in the nucleus of an atom — it identifies the element. All atoms of the same element have the same number of protons.
For neutral atoms, which have no overall charge, the number of electrons is the same as the number of protons. The number of neutrons is just mass number minus atomic number (top minus bottom in the nuclear symbol).
Ions -Atoms form ions by gaining or losing electrons. Ions have different numbers of protons and electrons — negative ions have more electrons than protons, and positive ions have fewer electrons than protons.
Negative ion: gain of electron(s)
Positive ion: loss of electron(s)
Isotopes of an element are atoms with the same number of protons but different numbers of neutrons. Isotopes have the same configuration of electrons, so they have the same chemical properties.
The relative atomic mass, Ar , is the average mass of an atom of an element on a scale where an atom of carbon-12 is exactly 12. The relative atomic mass of each element is shown in the periodic table.
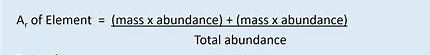
Mass Spectrometry:
The time of flight (TOF) mass spectrometer is an instrument used for measuring the masses of atoms and molecules. It can also be used to measure the relative abundance of different isotopes and to predict the structure of more complex molecules.

Ionisation The sample needs to be ionised before it enters the mass spectrometer. Two ways of doing this are:
Electrospray ionisation, — in this method the sample is dissolved in a solvent and pushed through a small nozzle at high pressure. A high voltage is applied to it, causing each particle to gain an H+ ion. The solvent is then removed, leaving a gas made up of positive ions.
Electron impact ionisation — in this method, the sample is vaporised and an ‘electron gun’ is used to fire high energy electrons at it. This knocks one electron off one each particle, so they become +1 ions.
Acceleration The positive ions are accelerated by an electric field. The electric field gives the same kinetic energy to all the ions. The lighter ions experience a greater acceleration — they’re given as much energy as the heavier ions, but they’re lighter, so they accelerate more.
Ion drift Next, the ions enter a region with no electric field. They drift through it at the same speed as they left the electric field. So, the lighter ions will be drifting at higher speeds.
Detection Because lighter ions travel through the drift region at higher speeds, they reach the detector in less time than heavier ions. The detector detects the current created when the ions hit it and records how long they took to pass through the spectrometer. This data is then used to calculate the mass/charge values needed to produce a mass spectrum.
Electron Arrangement:
Specification Points:
Electron configurations of atoms and ions up to Z = 36 in terms of shells and sub-shells (orbitals) s, p and d.
Ionisation energies.
Students should be able to:
define first ionisation energy
write equations for first and successive ionisation energies
explain how first and successive ionisation energies in Period 3 (Na–Ar) and in Group 2 (Be–Ba) give evidence for electron configuration in sub-shells and in shells.
In the currently accepted model of the atom, electrons have fixed energies. They move around the nucleus in certain regions of the atom called shells or energy levels. Each shell is given a number called the principal quantum number. The further a shell is from the nucleus, the higher its energy and the larger its principal quantum number.
Experiments show that not all the electrons in a shell have exactly the same energy. The atomic model explains this — shells are divided up into sub-shells. Different electron shells have different numbers of sub-shells, which each have a different energy. Sub-shells can be s sub-shells, p sub-shells, d sub-shells or f sub-shells.
The sub-shells have different numbers of orbitals which can each hold up to 2 electrons. The table on the right shows the number of orbitals in each sub-shell. You can use it to work out the number of electrons that each shell can hold.

The first ionisation energy is the energy needed to remove 1 electron from each atom in 1 mole of gaseous atoms to form 1 mole of gaseous 1+ ions.
Factors affecting ionisation energy:
A high ionisation energy means there’s a high attraction between the electron and the nucleus, so more energy is needed to remove the electron. There are three things that can affect ionisation energy:
==1 Nuclear charge-==the more protons there are in the nucleus, the more positively charged the nucleus is and the stronger the attraction for the electrons.
2 Distance from nucleus- Attraction falls off very rapidly with distance. An electron close to the nucleus will be much more strongly attracted than one further away.
==3 Shielding -==As the number of electrons between the outer electrons and the nucleus increases, the outer electrons feel less attraction to the nucleus. This lessening of the pull of the nucleus thanks to the inner electron shells is called shielding.
You can write equations for this process — here’s the equation for the first ionisation of oxygen:
O(g) +O (g) + e–
Here are a few rather important points about ionisation energies:
You must use the gas state symbol, (g), because ionisation energies are measured for gaseous atoms.
Always refer to 1 mole of atoms, as stated in the definition, rather than to a single atom.
The lower the ionisation energy, the easier it is to form a positive ion.
Ionisation trends down Group 2:
First ionisation energy decreases down Group 2.
This provides evidence that electron shells really exist. If each element going down Group 2 has one more electron shell than the one above, the extra shell will shield the outer electrons from the attraction of the nucleus. Also, the extra shell means that the outer electrons will be further from the nucleus, so the nucleus’s attraction will be reduced. It makes sense that both of these factors make it easier to remove outer electrons, resulting in lower ionisation energies.
Ionisation trends across periods:
The graph below shows the first ionisation energies of the elements in Period 3.
As you move across a period, the general trend is for the ionisation energies to increase — i.e. it gets harder to remove the outer electrons. This can be explained by the fact that the number of protons is increasing, which means a stronger nuclear attraction.
Ionisation energies and shell structure:
If you know the successive ionisation energies of an element you can work out the number of electrons in each shell of the atom and which group the element is in.
Within each shell, successive ionisation energies increase. This is because electrons are being removed from an increasingly positive ion — there’s less repulsion amongst the remaining electrons, so they’re held more strongly by the nucleus. The big jumps in ionisation energy happen when a new shell is broken into — an electron is being removed from a shell closer to the nucleus.
 Knowt
Knowt