
Chapter 7 - Quantum, Atomic, and Nuclear Physics
Basics
Einstein’s postulates of special relativity
All laws of physics remain the same in a uniformly moving frame of reference
The speed of light in a vacuum is always 3 x 10^8 no matter the motion of the source of light or the observer
Summary: time and distance are relative according to your frame of reference
E = mc^2
Mass is a solid form of energy and can be converted into energy and vice versa
Big 4 subatomic particles
Proton (p)
Mass = 1.67 x 10^-27 kg = 1 amu
Charge: positive
Electron (e)
Mass = 9.11 x 10^-31 kg
Charge: negative
Neutron (n)
Mass = 1.67 x 10^-27 kg = 1 amu
Charge: 0
Photon (ɣ)
Mass = 0
Charge: 0
Electron-Volts (eV)
Electron-Volt: a unit of energy - the amount of energy needed to change the potential of an electron by 1 volt
1 eV = 1.6 x 10^-17 J
Photons
Light is made of photons
E = hf = hc/λ
E: energy of a photon
h: Planck’s constant = 6.63 x 10^-34 Js = 4.14 x 10^-15 eVs
f: frequency (Hz)
c: speed of light (3 x 10^8 m/s)
λ: wavelength (m)
Photoelectric Effect
Applications: solar panels, photosynthesis, tanning, photographic film
Photoelectric effect: when incident light is shined on a metal, electrons detach
K(max) = hf - ɸ
K(max): max kinetic energy of the emitted electron
h: Planck’s constant
f: frequency
hf: energy of the incident photon
ɸ: work function - the energy required to remove an electron from a specific element/material
When the frequency of incident light increases, the maximum kinetic energy of the emitted electron increases linearly
Threshold frequency: minimum frequency for electron emission
Photon Momentum
When a photon collides with an atom and the atom emits an electron, momentum and energy are conserved
p = h/λ = E/c
DeBroglie Wavelength
If a particle has a shorter wavelength, it behaves more like a particle
If a particle has a longer wavelength, it behaves more like a wave
To find the wavelength for a particle (de Broglie’s wavelength), use λ = h/p = h/mv
λ: de Broglie’s wavelength
p: momentum of particle
Particles have a wave function representing the probability of finding the particle at a specific location
Ѱ: wave function
Ѱ = 0: no probability of finding the graph
Energy Levels in an atom
For an electron to move from one energy level to another, it will either have to absorb or emit energy in the form of a photon
The nucleus of an atom is positive and electrons are negative so it takes energy to pull the electron away from the nucleus by overcoming their attractive force
Electrons take less energy if they’re in a higher energy level
Key points
n1 is called the ground state - the lowest possible energy level for the electron
Moving from lower to higher energy levels tells you the atom absorbed a photon
Moving from higher to lower energy levels tells you the atom emitted a photon
There are no intermediate levels between energy levels
E (photon) = E (final) - E (initial)
If there is extra energy after jumping from one energy level to another, that energy is converted to kinetic energy of the emitted electron
Nuclear Decay
Particles involved with nuclear decay:
Alpha particle (𝞪): two protons and two neutrons together (helium nucleus)
Beta particle (β): either an electron or positron
Gamma particle (Ɣ): a gamma ray photon - massless and chargeless
Isotopes - same atomic number of an element but different mass numbers
Notation for Isotopes: element symbol with two small numbers to the left (one on top of the other)
Top number on the left side of the symbol: mass number = neutron # + proton #
Bottom number on the left side of the symbol: # of protons in the nucleus = atomic number
Alpha (𝞪) decay: a Helium nucleus is emitted from the original isotope
Beta (β) decay: either a positron or electron is emitted
β+ (also symbolized as e+): positron - +1 charge with negligible mass
β- (also symbolized as e-): electron - -1 charge with negligible mass
Gamma (Ɣ) decay: massless and chargeless photon
The photon carries away some energy and momentum so the nucleus recoils
__Neutron deca__y: a neutron is emitted
Mass defect: the slight difference in mass between the total mass present before the decay and after the decay
This difference in mass is destroyed and converted into kinetic energy
E = Δmc^2
Δm: mass defect
c: speed of light
E: energy produced
1 u = 931 MeV/c^2
The mass defect may become the nuclear binding energy and will be equal to the strong nuclear force that holds the nucleus together
Half-life: the time it takes for a radioactive isotope to decay half its original amount
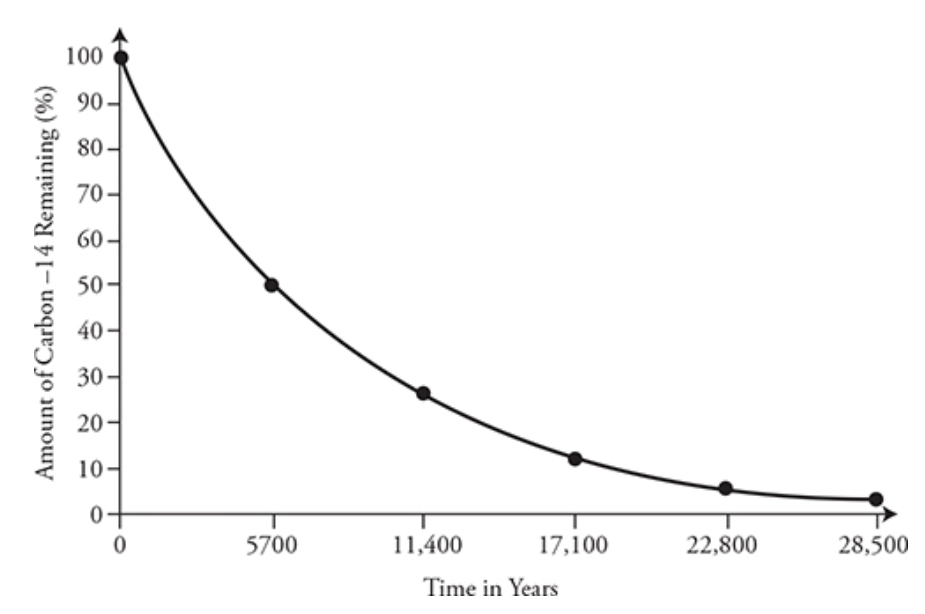
Longer half life → slow decay rate
Fission reactions: when a heavy nucleus is split into two chunks
Begun by shooting a neutron into the nucleus
Nuclear power plants and weapons
Fusion reactions: when two light nuclei combine to make a heavier and stable nucleus
Induced Reaction: scientists bombard a nucleus with high-speed particles to induce the emittance of a proton
Antimatter: every normal particle has an antimatter to match it (electron and positron)
When matter and antimatter meet, they annihilate each other
Ex: electron and positron can turn into photon energy
E (electron) + E(positron) = (2m)c^2 = hf
m: mass of electron
c: speed of light
h: Planck’s constant
f: frequency
Chapter 7 - Quantum, Atomic, and Nuclear Physics
Basics
Einstein’s postulates of special relativity
All laws of physics remain the same in a uniformly moving frame of reference
The speed of light in a vacuum is always 3 x 10^8 no matter the motion of the source of light or the observer
Summary: time and distance are relative according to your frame of reference
E = mc^2
Mass is a solid form of energy and can be converted into energy and vice versa
Big 4 subatomic particles
Proton (p)
Mass = 1.67 x 10^-27 kg = 1 amu
Charge: positive
Electron (e)
Mass = 9.11 x 10^-31 kg
Charge: negative
Neutron (n)
Mass = 1.67 x 10^-27 kg = 1 amu
Charge: 0
Photon (ɣ)
Mass = 0
Charge: 0
Electron-Volts (eV)
Electron-Volt: a unit of energy - the amount of energy needed to change the potential of an electron by 1 volt
1 eV = 1.6 x 10^-17 J
Photons
Light is made of photons
E = hf = hc/λ
E: energy of a photon
h: Planck’s constant = 6.63 x 10^-34 Js = 4.14 x 10^-15 eVs
f: frequency (Hz)
c: speed of light (3 x 10^8 m/s)
λ: wavelength (m)
Photoelectric Effect
Applications: solar panels, photosynthesis, tanning, photographic film
Photoelectric effect: when incident light is shined on a metal, electrons detach
K(max) = hf - ɸ
K(max): max kinetic energy of the emitted electron
h: Planck’s constant
f: frequency
hf: energy of the incident photon
ɸ: work function - the energy required to remove an electron from a specific element/material
When the frequency of incident light increases, the maximum kinetic energy of the emitted electron increases linearly
Threshold frequency: minimum frequency for electron emission
Photon Momentum
When a photon collides with an atom and the atom emits an electron, momentum and energy are conserved
p = h/λ = E/c
DeBroglie Wavelength
If a particle has a shorter wavelength, it behaves more like a particle
If a particle has a longer wavelength, it behaves more like a wave
To find the wavelength for a particle (de Broglie’s wavelength), use λ = h/p = h/mv
λ: de Broglie’s wavelength
p: momentum of particle
Particles have a wave function representing the probability of finding the particle at a specific location
Ѱ: wave function
Ѱ = 0: no probability of finding the graph
Energy Levels in an atom
For an electron to move from one energy level to another, it will either have to absorb or emit energy in the form of a photon
The nucleus of an atom is positive and electrons are negative so it takes energy to pull the electron away from the nucleus by overcoming their attractive force
Electrons take less energy if they’re in a higher energy level
Key points
n1 is called the ground state - the lowest possible energy level for the electron
Moving from lower to higher energy levels tells you the atom absorbed a photon
Moving from higher to lower energy levels tells you the atom emitted a photon
There are no intermediate levels between energy levels
E (photon) = E (final) - E (initial)
If there is extra energy after jumping from one energy level to another, that energy is converted to kinetic energy of the emitted electron
Nuclear Decay
Particles involved with nuclear decay:
Alpha particle (𝞪): two protons and two neutrons together (helium nucleus)
Beta particle (β): either an electron or positron
Gamma particle (Ɣ): a gamma ray photon - massless and chargeless
Isotopes - same atomic number of an element but different mass numbers
Notation for Isotopes: element symbol with two small numbers to the left (one on top of the other)
Top number on the left side of the symbol: mass number = neutron # + proton #
Bottom number on the left side of the symbol: # of protons in the nucleus = atomic number
Alpha (𝞪) decay: a Helium nucleus is emitted from the original isotope
Beta (β) decay: either a positron or electron is emitted
β+ (also symbolized as e+): positron - +1 charge with negligible mass
β- (also symbolized as e-): electron - -1 charge with negligible mass
Gamma (Ɣ) decay: massless and chargeless photon
The photon carries away some energy and momentum so the nucleus recoils
__Neutron deca__y: a neutron is emitted
Mass defect: the slight difference in mass between the total mass present before the decay and after the decay
This difference in mass is destroyed and converted into kinetic energy
E = Δmc^2
Δm: mass defect
c: speed of light
E: energy produced
1 u = 931 MeV/c^2
The mass defect may become the nuclear binding energy and will be equal to the strong nuclear force that holds the nucleus together
Half-life: the time it takes for a radioactive isotope to decay half its original amount

Longer half life → slow decay rate
Fission reactions: when a heavy nucleus is split into two chunks
Begun by shooting a neutron into the nucleus
Nuclear power plants and weapons
Fusion reactions: when two light nuclei combine to make a heavier and stable nucleus
Induced Reaction: scientists bombard a nucleus with high-speed particles to induce the emittance of a proton
Antimatter: every normal particle has an antimatter to match it (electron and positron)
When matter and antimatter meet, they annihilate each other
Ex: electron and positron can turn into photon energy
E (electron) + E(positron) = (2m)c^2 = hf
m: mass of electron
c: speed of light
h: Planck’s constant
f: frequency
 Knowt
Knowt
