
Physics - Electrostatics
Neutral v. Charged Objects
As discussed in a previous section of Lesson 1, atoms are the building blocks of matter. There are different types of atoms, known as elements. Atoms of each element are distinguished from each other by the number of protons that are present in their nucleus. An atom containing one proton is a hydrogen atom (H). An atom containing 6 protons is a carbon atom. And an atom containing 8 protons is an oxygen atom.
The number of electrons that surround the nucleus will determine whether or not an atom is electrically charged or electrically neutral. The amount of charge on a single proton is equal to the amount of charge possessed by a single electron. A proton and an electron have an equal amount but an opposite type of charge. Thus, if an atom contains equal numbers of protons and electrons, the atom is described as being electrically neutral. On the other hand, if an atom has an unequal number of protons and electrons, then the atom is electrically charged (and in fact, is then referred to as an ion rather than an atom). Any particle, whether an atom, molecule or ion, that contains less electrons than protons is said to be positively charged. Conversely, any particle that contains more electrons than protons is said to be negatively charged.
Charged versus Uncharged Particles | ||
Positively Charged | Negatively Charged | Uncharged |
Possesses more protons than electrons | Possesses more electrons than protons | Equal numbers of protons and electrons |
Charged Objects as an Imbalance of Protons and Electrons
In the previous section of Lesson 1, an atom was described as being a small and dense core of positively charged protons and neutral neutrons surrounded by shells of negatively charged electrons. The protons are tightly bound within the nucleus and not removable by ordinary measures. While the electrons are attracted to the protons of the nucleus, the addition of energy to an atom can persuade the electrons to leave an atom. Similarly, electrons within atoms of other materials can be persuaded to leave their own electron shells and become members of the electrons shells of other atoms of different materials. In short, electrons are migrants - constantly on the move and always ready to try out a new atomic environment.
 All objects are composed of these atoms. The electrons contained within the objects are prone to move or migrate to other objects. The process of an electron leaving one material object to reside (perhaps only temporarily) in another object is a common everyday occurrence. Even as you read the words of this web page, some electrons are likely moving through the monitor and adhering to your clothing (assuming that you are using this resource online) (and wearing clothes). If you were to walk across the carpeting towards the door of the room, electrons would likely be scuffed off the atoms of your shoes and moved onto the atoms of the carpet. And as clothes tumble in the dryer, it is highly likely that electrons on one piece of clothing will move from the atoms of the clothing onto the atoms of another piece of clothing. In general, for electrons to make a move from the atoms of one material to the atoms of another material, there must be an energy source, a motive, and a low-resistance pathway.
All objects are composed of these atoms. The electrons contained within the objects are prone to move or migrate to other objects. The process of an electron leaving one material object to reside (perhaps only temporarily) in another object is a common everyday occurrence. Even as you read the words of this web page, some electrons are likely moving through the monitor and adhering to your clothing (assuming that you are using this resource online) (and wearing clothes). If you were to walk across the carpeting towards the door of the room, electrons would likely be scuffed off the atoms of your shoes and moved onto the atoms of the carpet. And as clothes tumble in the dryer, it is highly likely that electrons on one piece of clothing will move from the atoms of the clothing onto the atoms of another piece of clothing. In general, for electrons to make a move from the atoms of one material to the atoms of another material, there must be an energy source, a motive, and a low-resistance pathway.
The cause and mechanisms by which this movement of electrons occurs will be the subject of Lesson 2. For now, it is sufficient to say that objects that are charged contain unequal numbers of protons and electrons. Charged objects have an imbalance of charge - either more negative electrons than positive protons or vice versa. And neutral objects have a balance of charge - equal numbers of protons and electrons. The principle stated earlier for atoms can be applied to objects. Objects with more electrons than protons are charged negatively; objects with fewer electrons than protons are charged positively.
In this discussion of electrically charged versus electrically neutral objects, the neutron has been neglected. Neutrons, being electrically neutral play no role in this unit. Their presence (or absence) will have no direct bearing upon whether an object is charged or uncharged. Their role in the atom is merely to provide stability to the nucleus, a subject not discussed in The Physics Classroom. When it comes to the drama of static electricity, electrons and protons become the main characters.
Charge as a Quantity
Like mass, the charge of an object is a measurable quantity. The charge possessed by an object is often expressed using the scientific unit known as the Coulomb. Just as mass is measured in grams or kilograms, charge is measured in units of Coulombs (abbreviated C). Because one Coulomb of charge is an abnormally large quantity of charge, the units of microCoulombs (µC) or nanoCoulombs (nC) are more commonly used as the unit of measurement of charge. To illustrate the magnitude of 1 Coulomb, an object would need an excess of 6.25 x 1018 electrons to have a total charge of -1 C. And of course an object with a shortage of 6.25 x 1018 electrons would have a total charge of +1 C.
The charge on a single electron is -1.6 x 10 -19 Coulomb. The charge on a single proton is +1.6 x 10 -19 Coulomb. The quantity of charge on an object reflects the amount of imbalance between electrons and protons on that object. Thus, to determine the total charge of a positively charged object (an object with an excess of protons), one must subtract the total number of electrons from the total number of protons. This operation yields the number of excess protons. Since a single proton contributes a charge of +1.6 x 10 -19 Coulomb to the overall charge of an atom, the total charge can be computed by multiplying the number of excess protons by +1.6 x 10 -19 Coulomb. A similar process is used to determine the total charge of a negatively charged object (an object with an excess of electrons), except that the number of protons is first subtracted from the number of electrons.
This principle is illustrated in the following table.
Object | # of Excess Protons/Electrons | Quantity and Kind of Charge (Q) on Object in Coulombs (C) |
A | 1 x 106 excess electrons | -1.6 x 10-13 C |
B | 1 x 106 excess protons | +1.6 x 10-13 C |
C | 2 x 1010 excess electrons | -3.2 x 10-9 C |
D | 3.5 x 108 excess protons | +5.6 x 10-11 C |
E | 4.67 x 1010 excess electrons | -7.5 x 10-9 C |
In conclusion, an electrically neutral object is an object that has a balance of protons and electrons. In contrast, a charged object has an imbalance of protons and electrons. Determining the quantity of charge on such an object involves a counting process; the total number of electrons and protons are compared to determine the difference between the number of protons and electrons. This difference is multiplied by 1.6 x 10 -19 Coulombs to determine the overall quantity of charge on the object. The type of charge (positive or negative) is determined by whether the protons or the electrons are in excess.
Charge Interactions
Suppose that you rubbed a balloon with a sample of animal fur such as a wool sweater or even your own hair. The balloon would likely become charged and its charge would exert a strange influence upon other objects in its vicinity. If some small bits of paper were placed upon a table and the balloon were brought near and held above the paper bits, then the presence of the charged balloon might create a sufficient attraction for the paper bits to raise them off the table. This influence - known as an electric force - occurs even when the charged balloon is held some distance away from the paper bits. The electric force is a non-contact force. Any charged object can exert this force upon other objects - both charged and uncharged objects. One goal of this unit of The Physics Classroom is to understand the nature of the electric force. In this part of Lesson 1, two simple and fundamental statements will be made and explained about the nature of the electric force.
Perhaps you have heard it said so many times that it sounds like a cliché.
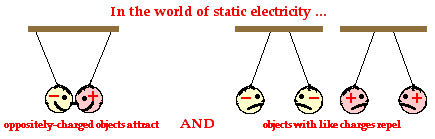
Opposites attract. And likes repel.
These two fundamental principles of charge interactions will be used throughout the unit to explain the vast array of static electricity phenomena. As mentioned in the previous section of Lesson 1, there are two types of electrically charged objects - those that contain more protons than electrons and are said to be positively charged and those that contain less protons than electrons and are said to be negatively charged. These two types of electrical charges - positive and negative - are said to be opposite types of charge. And consistent with our fundamental principle of charge interaction, a positively charged object will attract a negatively charged object. Oppositely charged objects will exert an attractive influence upon each other. In contrast to the attractive force between two objects with opposite charges, two objects that are of like charge will repel each other. That is, a positively charged object will exert a repulsive force upon a second positively charged object. This repulsive force will push the two objects apart. Similarly, a negatively charged object will exert a repulsive force upon a second negatively charged object. Objects with like charge repel each other.
The Electric Force and Newton's Third Law
This electric force exerted between two oppositely charged objects or two like charged objects is a force in the same sense that friction, tension, gravity and air resistance are forces. And being a force, the same laws and principles that describe any force describe the electrical force. One of those laws was Newton's law of action-reaction (discussed in Unit 2 of The Physics Classroom). According to Newton's third law, a force is simply a mutual interaction between two objects that results in an 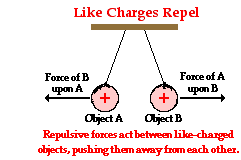 equal and opposite push or pull upon those objects. Let's apply Newton's third law to describe the interaction between Object A and Object B, both having positive charge.
equal and opposite push or pull upon those objects. Let's apply Newton's third law to describe the interaction between Object A and Object B, both having positive charge.
Object A exerts a rightward push upon Object B. Object B exerts a leftward push upon Object A. See diagram at right. These two pushing forces have equal magnitudes and are exerted in opposite directions of each other. Each object does its own pushing upon the other. The push upon Object B (by Object A) is directed away from Object A; and the push upon Object A (by Object B) is directed away from Object B. Because of the away from nature of the mutual interaction, the force is said to be repulsive.
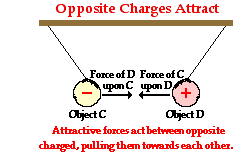 Now let's apply the same action-reaction principle to two oppositely charged objects - Object C (positive) and Object D (negative). See diagram at right. Object C exerts a leftward pull upon object D. Object D exerts a rightward pull upon Object C. Again, each object does its own pulling of the other. Just as before, these two forces have equal magnitudes and are exerted in opposite directions of each other. However in this instance, the direction of the force on Object D is towards Object C and the direction of the force on Object C is towards object D. Because of the towards each other nature of the mutual interaction, the force is described as being attractive.
Now let's apply the same action-reaction principle to two oppositely charged objects - Object C (positive) and Object D (negative). See diagram at right. Object C exerts a leftward pull upon object D. Object D exerts a rightward pull upon Object C. Again, each object does its own pulling of the other. Just as before, these two forces have equal magnitudes and are exerted in opposite directions of each other. However in this instance, the direction of the force on Object D is towards Object C and the direction of the force on Object C is towards object D. Because of the towards each other nature of the mutual interaction, the force is described as being attractive.
Interaction Between Charged and Neutral Objects
The interaction between two like-charged objects is repulsive. The interaction between two oppositely charged objects is attractive. What type of interaction is observed between a charged object and a neutral object? The answer is quite surprising to many students of physics. Any charged object - whether positively charged or negatively charged - will have an attractive interaction with a neutral object. Positively charged objects and neutral objects attract each other; and negatively charged objects and neutral objects attract each other.
 This third interaction between charged and neutral objects is often demonstrated by physics teachers or experienced by students in physics lab activities. For instance, if a charged balloon is held above neutral bits of paper, the force of attraction for the paper bits will be strong enough to overwhelm the downward force of gravity and raise the bits of paper off the table. If a charged plastic tube is held above some bits of paper, the tube will exert an attractive influence upon the paper to raise it off the table. And to the bewilderment of many, a charged rubber balloon can be attracted to a wooden cabinet with enough force that it sticks to the cabinet. Any charged object - plastic, rubber, or aluminum - will exert an attractive force upon a neutral object. And in accordance with Newton's law of action-reaction, the neutral object attracts the charged object.
This third interaction between charged and neutral objects is often demonstrated by physics teachers or experienced by students in physics lab activities. For instance, if a charged balloon is held above neutral bits of paper, the force of attraction for the paper bits will be strong enough to overwhelm the downward force of gravity and raise the bits of paper off the table. If a charged plastic tube is held above some bits of paper, the tube will exert an attractive influence upon the paper to raise it off the table. And to the bewilderment of many, a charged rubber balloon can be attracted to a wooden cabinet with enough force that it sticks to the cabinet. Any charged object - plastic, rubber, or aluminum - will exert an attractive force upon a neutral object. And in accordance with Newton's law of action-reaction, the neutral object attracts the charged object.
Flickr Physics Photo
A balloon is charged by rubbing it with hair. It is then brought near some bits of paper. The charged balloon attracts the paper bits, lifting them up off the table. This demonstrates the attraction between charged objects and neutral objects.
Repulsion versus Attraction
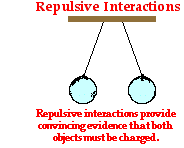 Because charged objects interact with their surroundings, an observed interaction provides possible evidence that an object is charged. Suppose that you enter the physics classroom and observe two balloons suspended from the ceiling. Rather than hanging straight down vertically, the balloons are hanging at an angle, exhibiting a repulsive interaction as shown at the right. The only way that two objects can repel each other is if they are both charged with the same type of charge. Thus, the repulsion of the balloons provides conclusive evidence that both balloons are charged and charged with the same type of charge. One could not conclude that the balloons are both positively charged or both negatively charged. Additional information or further testing would be required to make a conclusion about the type of excess charge present upon the balloons. Nonetheless, one can be convinced that both balloons possess an excess charge - either positive or negative.
Because charged objects interact with their surroundings, an observed interaction provides possible evidence that an object is charged. Suppose that you enter the physics classroom and observe two balloons suspended from the ceiling. Rather than hanging straight down vertically, the balloons are hanging at an angle, exhibiting a repulsive interaction as shown at the right. The only way that two objects can repel each other is if they are both charged with the same type of charge. Thus, the repulsion of the balloons provides conclusive evidence that both balloons are charged and charged with the same type of charge. One could not conclude that the balloons are both positively charged or both negatively charged. Additional information or further testing would be required to make a conclusion about the type of excess charge present upon the balloons. Nonetheless, one can be convinced that both balloons possess an excess charge - either positive or negative.
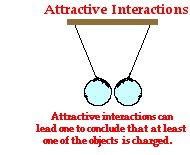 Now let's contrast the observation of repulsion with that of attraction. Suppose that you now enter the physics classroom and observe two balloons suspended from the ceiling and exhibiting an attractive interaction as shown at the right. There are two underlying reasons for two objects attracting each other. One balloon could be neutral and the other balloon charged or both balloons could be charged with the opposite type of charge. Thus, your only conclusion could be that at least one of the balloons is charged. The other balloon is either neutral or charged with the opposite type of charge. You cannot draw a conclusion about which one of the balloons is charged or what type of charge (positive or negative) the charged balloon possesses. Additional information or further testing would be required to make these conclusions. For example, if you could take each balloon and individually bring them near some neutral bits of paper, you could test to see if each individual balloon is charged or neutral. If a balloon were charged, then it would exhibit an attractive interaction with the neutral paper bits. On the other hand, an uncharged balloon would not interact at all with neutral paper bits.
Now let's contrast the observation of repulsion with that of attraction. Suppose that you now enter the physics classroom and observe two balloons suspended from the ceiling and exhibiting an attractive interaction as shown at the right. There are two underlying reasons for two objects attracting each other. One balloon could be neutral and the other balloon charged or both balloons could be charged with the opposite type of charge. Thus, your only conclusion could be that at least one of the balloons is charged. The other balloon is either neutral or charged with the opposite type of charge. You cannot draw a conclusion about which one of the balloons is charged or what type of charge (positive or negative) the charged balloon possesses. Additional information or further testing would be required to make these conclusions. For example, if you could take each balloon and individually bring them near some neutral bits of paper, you could test to see if each individual balloon is charged or neutral. If a balloon were charged, then it would exhibit an attractive interaction with the neutral paper bits. On the other hand, an uncharged balloon would not interact at all with neutral paper bits.
The above thought experiments illustrate the conclusive nature of a repulsive interaction. When objects repel each other, one can be certain that both objects are charged. On the other had, the observation of an attractive interaction leads to limited conclusions. At best, one can conclude that at least one of the objects is charged.
Conductors and Insulators
The behavior of an object that has been charged is dependent upon whether the object is made of a conductive or a nonconductive material. Conductors are materials that permit electrons to flow freely from particle to particle. An object made of a conducting material will permit charge to be transferred across the entire surface of the object. If charge is transferred to the object at a given location, that charge is quickly distributed across the entire surface of the object. The distribution of charge is the result of electron movement. Since conductors allow for electrons to be transported from particle to particle, a charged object will always distribute its charge until the overall repulsive forces between excess electrons is minimized. If a charged conductor is touched to another object, the conductor can even transfer its charge to that object. The transfer of charge between objects occurs more readily if the second object is made of a conducting material. Conductors allow for charge transfer through the free movement of electrons.
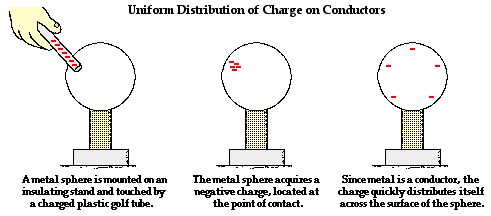
In contrast to conductors, insulators are materials that impede the free flow of electrons from atom to atom and molecule to molecule. If charge is transferred to an insulator at a given location, the excess charge will remain at the initial location of charging. The particles of the insulator do not permit the free flow of electrons; subsequently charge is seldom distributed evenly across the surface of an insulator.
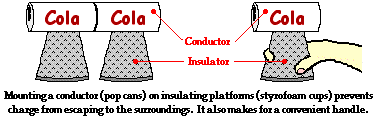
While insulators are not useful for transferring charge, they do serve a critical role in electrostatic experiments and demonstrations. Conductive objects are often mounted upon insulating objects. This arrangement of a conductor on top of an insulator prevents charge from being transferred from the conductive object to its surroundings. This arrangement also allows for a student (or teacher) to manipulate a conducting object without touching it. The insulator serves as a handle for moving the conductor around on top of a lab table. If charging experiments are performed with aluminum pop cans, then the cans should be mounted on top of Styrofoam cups. The cups serve as insulators, preventing the pop cans from discharging their charge. The cups also serve as handles when it becomes necessary to move the cans around on the table.
Examples of Conductors and Insulators
Examples of conductors include metals, aqueous solutions of salts (i.e., ionic compounds dissolved in water), graphite, and the human body. Examples of insulators include plastics, Styrofoam, paper, rubber, glass and dry air. The division of materials into the categories of conductors and insulators is a somewhat artificial division. It is more appropriate to think of materials as being placed somewhere along a continuum. Those materials that are super conductive (known as superconductors) would be placed at on end and the least conductive materials (best insulators) would be placed at the other end. Metals would be placed near the most conductive end and glass would be placed on the opposite end of the continuum. The conductivity of a metal might be as much as a million trillion times greater than that of glass.

Along the continuum of conductors and insulators, one might find the human body somewhere towards the conducting side of the middle. When  the body acquires a static charge it has a tendency to distribute that charge throughout the surface of the body. Given the size of the human body, relative to the size of typical objects used in electrostatic experiments, it would require an abnormally large quantity of excess charge before its effect is noticeable. The effects of excess charge on the body are often demonstrated using a Van de Graaff generator. When a student places their hand upon the static ball, excess charge from the ball is shared with the human body. Being a conductor, the excess charge could flow to the human body and spread throughout the surface of the body, even onto strands of hair. As the individual strands of hair become charged, they begin to repel each other. Looking to distance themselves from their like-charged neighbors, the strands of hair begin to rise upward and outward - a truly hair-raising experience.
the body acquires a static charge it has a tendency to distribute that charge throughout the surface of the body. Given the size of the human body, relative to the size of typical objects used in electrostatic experiments, it would require an abnormally large quantity of excess charge before its effect is noticeable. The effects of excess charge on the body are often demonstrated using a Van de Graaff generator. When a student places their hand upon the static ball, excess charge from the ball is shared with the human body. Being a conductor, the excess charge could flow to the human body and spread throughout the surface of the body, even onto strands of hair. As the individual strands of hair become charged, they begin to repel each other. Looking to distance themselves from their like-charged neighbors, the strands of hair begin to rise upward and outward - a truly hair-raising experience.
Many are familiar with the impact that humidity can have upon static charge buildups. You have likely noticed that bad hair days, doorknob shocks and static clothing are most common during winter months. Winter months tend to be the driest months of the year with humidity levels in the air dropping to lower values. Water has a tendency to gradually remove excess charge from objects. When the humidity is high, a person acquiring an excess charge will tend to lose that charge to water molecules in the surrounding air. On the other hand, dry air conditions are more conducive to the buildup of static charge and more frequent electric shocks. Since humidity levels tend to vary from day to day and season to season, it is expected that electrical effects (and even the success of electrostatic demonstrations) can vary from day to day.
Distribution of Charge via Electron Movement
Predicting the direction that electrons would move within a conducting material is a simple application of the two fundamental rules of charge interaction. Opposites attract and likes repel. Suppose that some method is used to impart a negative charge to an object at a given location. At the location where the charge is imparted, there is an excess of electrons. That is, the multitude of atoms in that region possess more electrons than protons. Of course, there are a number of electrons that could be thought of as being quite contented since there is an accompanying positively charged proton to satisfy their attraction for an opposite. However, the so-called excess electrons have a repulsive response to each other and would prefer more space. Electrons, like human beings, wish to manipulate their surroundings in an effort to reduce repulsive effects. Since these excess electrons are present in a conductor, there is little hindrance to their ability to migrate to other parts of the object. And that is exactly what they do. In an effort to reduce the overall repulsive effects within the object, there is a mass migration of excess electrons throughout the entire surface of the object. Excess electrons migrate to distance themselves from their repulsive neighbors. In this sense, it is said that excess negative charge distributes itself throughout the surface of the conductor.
But what happens if the conductor acquires an excess of positive charge? What if electrons are removed from a conductor at a given location, giving the object an overall positive charge? If protons cannot move, then how can the excess of positive charge distribute itself across the surface of the material? While the answers to these questions are not as obvious, it still involves a rather simple explanation that once again relies on the two fundamental rules of charge interaction. Opposites attract and likes repel. Suppose that a conducting metal sphere is charged on its left side and imparted an excess of positive charge. (Of course, this requires that electrons be removed from the object at the location of charging.) A multitude of atoms in the region where the charging occurs have lost one or more electrons and have an excess of protons. The imbalance of charge within these atoms creates effects that can be thought of as disturbing the balance of charge within the entire object. The presence of these excess protons in a given location draws electrons from other atoms. Electrons in other parts of the object can be thought of as being quite contented with the balance of charge that they are experiencing. Yet there will always be some electrons that will feel the attraction for the excess protons some distance away. In human terms, we might say these electrons are drawn by curiosity or by the belief that the grass is greener on the other side of the fence. In the language of electrostatics, we simply assert that opposites attract - the excess protons and both the neighboring and distant electrons attract each other. The protons cannot do anything about this attraction since they are bound within the nucleus of their own atoms. Yet, electrons are loosely bound within atoms; and being present in a conductor, they are free to move. These electrons make the move for the excess protons, leaving their own atoms with their own excess of positive charge. This electron migration happens across the entire surface of the object, until the overall sum of repulsive effects between electrons across the whole surface of the object are minimized.
Polarization
Inducing the Movement of Charge
As discussed previously, an atom consists of positively charged protons and negatively charged electrons. The protons are in the nucleus of the atom, tightly bound and incapable of movement. The electrons are located in the vast regions of space surrounding the nucleus, known as the electron shells or the electron clouds. Relative to the protons of the nucleus, these electrons are loosely bound. In conducting objects, they are so loosely bound that they may be induced into moving from one portion of the object to another portion of the object. To get an electron in a conducting object to get up and go, all that must be done is to place a charged object nearby the conducting object.
To illustrate this induced movement of electrons, we will consider an aluminum pop can that is taped to a Styrofoam cup. The Styrofoam cup serves as both an insulating stand and a handle. A rubber balloon is charged negatively, perhaps by rubbing it against animal fur. If the negatively charged balloon is brought near the aluminum pop can, the electrons within the pop can will experience a repulsive force. The repulsion will be greatest for those electrons that are nearest the negatively charged balloon. Many of these electrons will be induced into moving away from the repulsive balloon. Being present within a conducting material, the electrons are free to move from atom to atom. As such, there is a mass migration of electrons from the balloon's side of the aluminum can towards the opposite side of the can. This electron movement leaves atoms on the balloon's side of the can with a shortage of electrons; they become positively charged. And the atoms on the side opposite of the can have an excess of electrons; they become negatively charged. The two sides of the aluminum pop can have opposite charges. Overall the can is electrically neutral; it's just that the positive and negative charge has been separated from each other. We say that the charge in the can has been polarized.
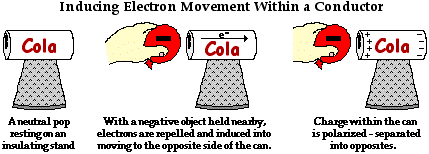
In general terms, polarization means to separate into opposites. In the political world, we often observe that a collection of people becomes polarized over some issue. For instance, we might say that the United States has become polarized over the issue of the death penalty. That is, the citizens of the United States have been separated into opposites - those who are for the death penalty and those who are against the death penalty. In the context of electricity, polarization is the process of separating opposite charges within an object. The positive charge becomes separated from the negative charge. By inducing the movement of electrons within an object, one side of the object is left with an excess of positive charge and the other side of the object is left with an excess of negative charge. Charge becomes separated into opposites.
The polarization process always involves the use of a charged object to induce electron movement or electron rearrangement. In the above diagram and accompanying discussion, electrons within a conducting object were induced into moving from the left side of the conducting can to the right side of the can. Being a conductor, electrons were capable of moving from atom to atom across the entire surface of the conductor. But what if the object being polarized is an insulator? Electrons are not free to move across the surface of an insulator. How can an insulator such as a wooden wall be polarized?
How Can an Insulator be Polarized?
Polarization can occur within insulators, but the process occurs in a different manner than it does within a conductor. In a conducting object, electrons are induced into movement across the surface of the conductor from one side of the object to the opposite side. In an insulator, electrons merely redistribute themselves within the atom or molecules nearest the outer surface of the object. To understand the electron redistribution process, it is important to take another brief excursion into the world of atoms, molecules and chemical bonds.
The electrons surrounding the nucleus of an atom are believed to be located in regions of space with specific shapes and sizes. The actual size and shape of these regions is determined by the high-powered mathematical equations common to Quantum Mechanics. Rather than being located a specific distance from the nucleus in a fixed orbit, the electrons are simply thought of as being located in regions often referred to as electron clouds. At any given moment, the electron is likely to be found at some location within the cloud. The electron clouds have varying density; the density of the cloud is considered to be greatest in the portion of the cloud where the electron has the greatest probability of being found at any given moment. And conversely, the electron cloud density is least in the regions where the electron is least likely to be found. In addition to having varying density, these electron clouds are also highly distortable. The presence of neighboring atoms with high electron affinity can distort the electron clouds around atoms. Rather than being located symmetrically about the positive nucleus, the cloud becomes asymmetrically shaped. As such, there is a polarization of the atom as the centers of positive and negative charge are no longer located in the same location. The atom is still a neutral atom; it has just become polarized.

The discussion becomes even more complex (and perhaps too complex for our purposes) when we consider molecules - combination of atoms bonded together. In molecules, atoms are bonded together as protons in one atom attract the electrons in the clouds of another atom. This electrostatic attraction results in a bond between the two atoms. Electrons are shared by the two atoms as they begin to overlap their electron clouds. If the atoms are of different types (for instance, one atom is Hydrogen and the other atom is Oxygen), then the electrons within the clouds of the two atoms are not equally  shared by the atoms. The clouds become distorted, with the electrons having the greatest probability of being found closest to the more electron-greedy atom. The bond is said to be a polar bond. The distribution of electrons within the cloud is shifted more towards one atom than towards the other atom. This is the case for the two hydrogen-oxygen bonds in the water molecule. Electrons shared by these two atoms are drawn more towards the oxygen atom than towards the hydrogen atom. Subsequently, there is a separation of charge, with oxygen having a partially negative charge and hydrogen having a partially positive charge.
shared by the atoms. The clouds become distorted, with the electrons having the greatest probability of being found closest to the more electron-greedy atom. The bond is said to be a polar bond. The distribution of electrons within the cloud is shifted more towards one atom than towards the other atom. This is the case for the two hydrogen-oxygen bonds in the water molecule. Electrons shared by these two atoms are drawn more towards the oxygen atom than towards the hydrogen atom. Subsequently, there is a separation of charge, with oxygen having a partially negative charge and hydrogen having a partially positive charge.
It is very common to observe this polarization within molecules. In molecules that have long chains of atoms bonded together, there are often several locations along the chain or near the ends of the chain that have polar bonds. This polarization leaves the molecule with areas that have a concentration of positive charges and other areas with a concentration of negative charges. This principle is utilized in the manufacture of certain commercial products that are used to reduce static cling. The centers of positive and negative charge within the product are drawn to excess charge residing on the clothes. There is a neutralization of the static charge buildup on the clothes, thus reducing their tendency to be attracted to each other. (Other products actually use a different principle. During manufacturing, a thin sheet is soaked in a solution containing positively charged ions. The sheet is tossed into the dryer with the clothes. Being saturated with positive charges, the sheet is capable of attracting excess electrons that are scuffed off of clothes during the drying cycle.)
How Does Polarization Explain the Balloon and the Wall Demonstration?
A complete discussion of the world of atoms, molecules and chemical bonds is beyond the scope of The Physics Classroom. Nonetheless, a model of the atom as a distortable cloud of negative electrons surrounding a positive nucleus becomes essential to understanding how an insulating material can be polarized. If a charged object is brought near an insulator, the charges on that object are capable of distorting the electron clouds of the insulator atoms. There is a polarization of the neutral atoms. As shown in the diagrams below, the neutral atoms of the insulator will orient themselves in such a manner as to place the more attractive charge nearest the charged object. Once polarized in this manner, opposites can now attract.

A common demonstration performed in class involved bringing a negatively charged balloon near a wooden door or wooden cabinet. The molecules of wood will reorient themselves in such a way as to place their  positive charges towards the negatively charged balloon. The distortion of their electron clouds will result in an alignment of the wood molecules in a manner that makes the wooden cabinet attracted to the negatively charged balloon. In human terms, one might say that the wood does some quick grooming and then places its most attractive side towards the balloon and its most repulsive side away from the balloon. In the world of static electricity, closeness counts. The negative balloon is closer to the positive portion of the wood molecules and further from the more repulsive negative portion. The balloon and the wall attract with sufficient force to cause the balloon to stick to the wall. From a mechanics standpoint, we would say that the balloon and the wall are pressed together with a large force. The large normal force on the balloon results in a large static friction force. This friction force balances the downward force of gravity and the balloon remains at rest.
positive charges towards the negatively charged balloon. The distortion of their electron clouds will result in an alignment of the wood molecules in a manner that makes the wooden cabinet attracted to the negatively charged balloon. In human terms, one might say that the wood does some quick grooming and then places its most attractive side towards the balloon and its most repulsive side away from the balloon. In the world of static electricity, closeness counts. The negative balloon is closer to the positive portion of the wood molecules and further from the more repulsive negative portion. The balloon and the wall attract with sufficient force to cause the balloon to stick to the wall. From a mechanics standpoint, we would say that the balloon and the wall are pressed together with a large force. The large normal force on the balloon results in a large static friction force. This friction force balances the downward force of gravity and the balloon remains at rest.
Another common physics (and chemistry) demonstration involves using a charged object to deflect a stream of water from its path. Most often, a comb is charged negatively by combing one's hair or a rubber balloon is charged in a similar manner. The negatively charged object is then brought near to a falling stream of water, causing the stream to be attracted to the comb or balloon and alter its direction of fall. The demonstration illustrates the polar nature of water molecules. The hydrogen atoms serve as the positive poles within a water molecule; oxygen serves as the negative pole. Molecules of a liquid are free to rotate and move about; the water molecules realign themselves in order to put their positive poles towards the negatively charged object. Once polarized, the stream and the balloon (or comb) are attracted. As the water molecules within the stream fall past the balloon, this realignment of individual molecules happens quickly and the entire stream is deflected from its original downward direction.
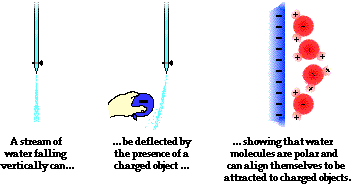
Examples of the attraction between charged objects and neutral objects are numerous and often demonstrated by physics teachers. Paper bits become polarized and are attracted to a charged piece of acetate. Small penguins cut from a sheet of paper are attracted to a charged plastic golf tube and demonstrate their happy feet. A long wooden 2x4 is placed on a pivot and becomes polarized and attracted to a charged golf tube. To the astonishment of students, the force of attraction on the wood is large enough to rotate it about the pivot point.
Polarization is Not Charging
Perhaps the biggest misconception that pertains to polarization is the belief that polarization involves the charging of an object. Polarization is not charging! When an object becomes polarized, there is  simply a redistribution of the centers of positive and negative charges within the object. Either by the movement of electrons across the surface of the object (as is the case in conductors) or through the distortion of electron clouds (as is the case in insulators), the centers of positive and negative charges become separated from each other. The atoms at one location on the object possess more protons than electrons and the atoms at another location have more electrons than protons. While there are the same number of protons and electrons within the object, these protons and electrons are not distributed in the same proportion across the object's surface. Yet, there are still equal numbers of positive charges (protons) and negative charges (electrons) within the object. While there is a separation of charge, there is NOT an imbalance of charge. When neutral objects become polarized, they are still neutral objects.
simply a redistribution of the centers of positive and negative charges within the object. Either by the movement of electrons across the surface of the object (as is the case in conductors) or through the distortion of electron clouds (as is the case in insulators), the centers of positive and negative charges become separated from each other. The atoms at one location on the object possess more protons than electrons and the atoms at another location have more electrons than protons. While there are the same number of protons and electrons within the object, these protons and electrons are not distributed in the same proportion across the object's surface. Yet, there are still equal numbers of positive charges (protons) and negative charges (electrons) within the object. While there is a separation of charge, there is NOT an imbalance of charge. When neutral objects become polarized, they are still neutral objects.
The process of polarization is often used in many charging methods. In one section of Lesson 2, the charging by induction process will be discussed. This charging process depends upon a charged object to induce polarization within a neutral object. While charging by induction includes polarization as one of its steps, polarization is still NOT a charging process. Details about the induction charging method can be read about in Lesson 2 of this unit.
Triboelectric Charging
How Triboelectric Charging Works
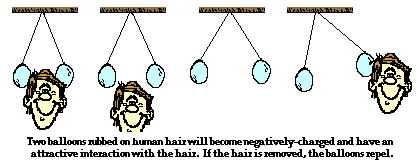
The triboelectic charging process (a.k.a., charging by friction) results in a transfer of electrons between the two objects that are rubbed together. Rubber has a much greater attraction for electrons than animal fur. As a result, the atoms of rubber pull electrons from the atoms of animal fur, leaving both objects with an imbalance of charge. The rubber balloon has an excess of electrons and the animal fur has a shortage of electrons. Having an excess of electrons, the rubber balloon is charged negatively. Similarly, the shortage of electrons on the animal fur leaves it with a positive charge. The two objects have become charged with opposite types of charges as a result of the transfer of electrons from the least electron-loving material to the most electron-loving material.
Triboelectic charging is often demonstrated in Physics class. Two rubber balloons can be suspended from the ceiling and hung at approximately head height. When rubbed upon a teacher's head, the balloons became charged as electrons are transferred from the teacher's fur to the balloons. Since the teacher's fur lost electrons, it became positively charged and the subsequent attraction between the two rubbed objects could be observed. Of course, when the teacher pulls away from the balloons, the balloons experienced a repulsive interaction for each other.
 As mentioned, different materials have different affinities for electrons. By rubbing a variety of materials against each other and testing their resulting interaction with objects of known charge, the tested materials can be ordered according to their affinity for electrons. Such an ordering of substances is known as a triboelectric series. One such ordering for several materials is shown in the table at the right. Materials shown highest on the table tend to have a greater affinity for electrons than those below it. Subsequently, when any two materials in the table are rubbed together, the one that is higher can be expected to pull electrons from the material that is lower. As such, the materials highest on the table will have the greatest tendency to acquire the negative charge. Those below it would become positively charged.
As mentioned, different materials have different affinities for electrons. By rubbing a variety of materials against each other and testing their resulting interaction with objects of known charge, the tested materials can be ordered according to their affinity for electrons. Such an ordering of substances is known as a triboelectric series. One such ordering for several materials is shown in the table at the right. Materials shown highest on the table tend to have a greater affinity for electrons than those below it. Subsequently, when any two materials in the table are rubbed together, the one that is higher can be expected to pull electrons from the material that is lower. As such, the materials highest on the table will have the greatest tendency to acquire the negative charge. Those below it would become positively charged.
The Law of Conservation of Charge
The triboelectic charging process (as well as any charging process) involves a transfer of electrons between two objects. Charge is not created from nothing. The appearance of negative charge upon a rubber balloon is merely the result of its acquisition of electrons. And these electrons must come from somewhere; in this case, from the object it was rubbed against. Electrons are transferred in any charging process. In the case of triboelectic charging, they are transferred between the two objects being rubbed together. Prior to the charging, both objects are electrically neutral. The net charge of the system is 0 units. After the charging process, the more electron-loving object may acquire a charge of -12 units; the other object acquires a charge of +12 units. Overall, the system of two objects has a net charge of 0 units. Whenever a quantity like charge (or momentum or energy or matter) is observed to be the same prior to and after the completion of a given process, we say that the quantity is conserved. Charge is always conserved. When all objects involved are considered prior to and after a given process, we notice that the total amount of charge amidst the objects is the same before the process starts as it is after the process ends. This is referred to as the law of conservation of charge.
Charging By Induction
Charging a Two-Sphere System Using a Negatively Charged Object
 One common demonstration performed in a physics classroom involves the induction charging of two metal spheres. The metal spheres are supported by insulating stands so that any charge acquired by the spheres cannot travel to the ground. The spheres are placed side by side (see diagram i. below) so as to form a two-sphere system. Being made of metal (a conductor), electrons are free to move between the spheres - from sphere A to sphere B and vice versa. If a rubber balloon is charged negatively (perhaps by rubbing it with animal fur) and brought near the spheres, electrons within the two-sphere system will be induced to move away from the balloon. This is simply the principle that like charges repel. Being charged negatively, the electrons are repelled by the negatively charged balloon. And being present in a conductor, they are free to move about the surface of the conductor. Subsequently, there is a mass migration of electrons from sphere A to sphere B. This electron migration causes the two-sphere system to be polarized (see diagram ii. below). Overall, the two-sphere system is electrically neutral. Yet the movement of electrons out of sphere A and into sphere B separates the negative charge from the positive charge. Looking at the spheres individually, it would be accurate to say that sphere A has an overall positive charge and sphere B has an overall negative charge. Once the two-sphere system is polarized, sphere B is physically separated from sphere A using the insulating stand. Having been pulled further from the balloon, the negative charge likely redistributes itself uniformly about sphere B (see diagram iii. below). Meanwhile, the excess positive charge on sphere A remains located near the negatively charged balloon, consistent with the principle that opposite charges attract. As the balloon is pulled away, there is a uniform distribution of charge about the surface of both spheres (see diagram iv. below). This distribution occurs as the remaining electrons in sphere A move across the surface of the sphere until the excess positive charge is uniformly distributed. (This distribution of positive charge on a conductor was discussed in detail earlier in Lesson 1.)
One common demonstration performed in a physics classroom involves the induction charging of two metal spheres. The metal spheres are supported by insulating stands so that any charge acquired by the spheres cannot travel to the ground. The spheres are placed side by side (see diagram i. below) so as to form a two-sphere system. Being made of metal (a conductor), electrons are free to move between the spheres - from sphere A to sphere B and vice versa. If a rubber balloon is charged negatively (perhaps by rubbing it with animal fur) and brought near the spheres, electrons within the two-sphere system will be induced to move away from the balloon. This is simply the principle that like charges repel. Being charged negatively, the electrons are repelled by the negatively charged balloon. And being present in a conductor, they are free to move about the surface of the conductor. Subsequently, there is a mass migration of electrons from sphere A to sphere B. This electron migration causes the two-sphere system to be polarized (see diagram ii. below). Overall, the two-sphere system is electrically neutral. Yet the movement of electrons out of sphere A and into sphere B separates the negative charge from the positive charge. Looking at the spheres individually, it would be accurate to say that sphere A has an overall positive charge and sphere B has an overall negative charge. Once the two-sphere system is polarized, sphere B is physically separated from sphere A using the insulating stand. Having been pulled further from the balloon, the negative charge likely redistributes itself uniformly about sphere B (see diagram iii. below). Meanwhile, the excess positive charge on sphere A remains located near the negatively charged balloon, consistent with the principle that opposite charges attract. As the balloon is pulled away, there is a uniform distribution of charge about the surface of both spheres (see diagram iv. below). This distribution occurs as the remaining electrons in sphere A move across the surface of the sphere until the excess positive charge is uniformly distributed. (This distribution of positive charge on a conductor was discussed in detail earlier in Lesson 1.)
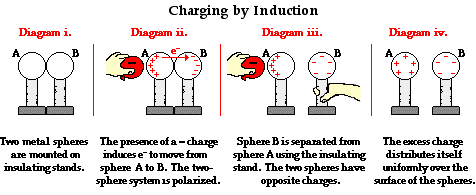
The Law of Conservation of Charge
The law of conservation of charge is easily observed in the induction charging process. Considering the example above, one can look at the two spheres as a system. Prior to the charging process, the overall charge of the system was zero. There were equal numbers of protons and electrons within the two spheres. In diagram ii. above, electrons were induced into moving from sphere A to sphere B. At this point, the individual spheres become charged. The quantity of positive charge on sphere A equals the quantity of negative charge on sphere B. If sphere A has 1000 units of positive charge, then sphere B has 1000 units of negative charge. Determining the overall charge of the system is easy arithmetic; it is simply the sum of the charges on the individual spheres.
Overall Charge of Two Spheres = +1000 units + (-1000 units) = 0 units
The overall charge on the system of two objects is the same after the charging process as it was before the charging process. Charge is neither created nor destroyed during this charging process; it is simply transferred from one object to the other object in the form of electrons.
Charging a Two-Sphere System Using a Positively Charged Object
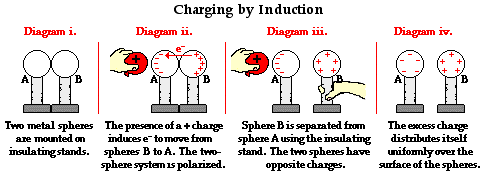
The above examples show how a negatively charged balloon is used to polarize a two-sphere system and ultimately charge the spheres by induction. But what would happen to sphere A and sphere B if a positively charged object was used to first polarize the two-sphere system? How would the outcome be different and how would the electron movement be altered?
Consider the graphic below in which a positively charged balloon is brought near Sphere A. The presence of the positive charge induces a mass migration of electrons from sphere B towards (and into) sphere A. This movement is induced by the simple principle that opposites attract. Negatively charged electrons throughout the two-sphere system are attracted to the positively charged balloon. This movement of electrons from sphere B to sphere A leaves sphere B with an overall positive charge and sphere A with an overall negative charge. The two-sphere system has been polarized. With the positively charged balloon still held nearby, sphere B is physically separated from sphere A. The excess positive charge is uniformly distributed across the surface of sphere B. The excess negative charge on sphere A remains crowded towards the left side of the sphere, positioning itself close to the balloon. Once the balloon is removed, electrons redistribute themselves about sphere A until the excess negative charge is evenly distributed across the surface. In the end, sphere A becomes charged negatively and sphere B becomes charged positively.
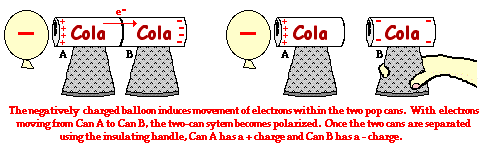
This induction charging process can be used to charge a pair of pop cans. It is a simple enough experiment to be repeated at home. Two pop cans are mounted on Styrofoam cups using scotch tape. The cans are placed side-by-side and a negatively charged rubber balloon (having been rubbed with animal fur) is brought near to one of the cans. The presence of the negative charge near a can induces electron movement from Can A to Can B (see diagram). Once the cans are separated, the cans are charged. The type of charge on the cans can be tested by seeing if they attract the negatively charged balloon or repel the negatively charged balloon. Of course, we would expect that Can A (being positively charged) would attract the negatively charged balloon and Can B (being negatively charged) should repel the negatively charged balloon. During the process of induction charging, the role of the balloon is to simply induce a movement of electrons from one can to the other can. It is used to polarize the two-can system. The balloon never does supply electrons to can A (unless your hear a spark, indicating a lightning discharge from the balloon to the can).
The Importance of a Ground in Induction Charging
In the charging by induction cases discussed above, the ultimate charge on the object is never the result of electron movement from the charged object to the originally neutral objects. The balloon never transfers electrons to or receive electrons from the spheres; nor does the glass rod transfer electrons to or receive electrons from the spheres. The neutral object nearest the charged object (sphere A in these discussions) acquires its charge from the object to which it is touched. In the above cases, the second sphere is used to supply the electrons to sphere A or to receive electrons from sphere A. The role of sphere B in the above examples is to serve as a supplier or receiver of electrons in response to the object that is brought near sphere A. In this sense, sphere B acts like a ground.
To further illustrate the importance of a ground, consider the induction charging of a single conducting sphere. Suppose that a negatively charged rubber balloon is brought near a single sphere as shown below (Diagram ii). The presence of the negative charge will induce electron movement in the sphere. Since like charges repel, negative electrons within the metal sphere will be repelled by the negatively charged balloon. There will be a mass migration of electrons from the left side of the sphere to the right side of the sphere causing charge within the sphere to become polarized (Diagram ii). Once charge within the sphere has become polarized, the sphere is touched. The touching of the sphere allows electrons to exit the sphere and move through the hand to "the ground" (Diagram iii). It is at this point that the sphere acquires a charge. With electrons having left the sphere, the sphere acquires a positive charge (Diagram iv). Once the balloon is moved away from the sphere, the excess positive charge redistributes itself (by the movement of remaining electrons) such that the positive charge is uniformly distributed about the sphere's surface.
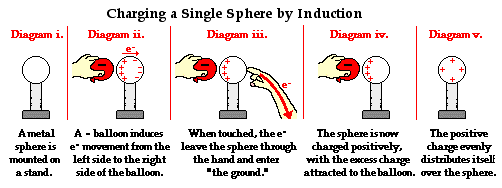
There are several things to note about this example of induction charging. First, observe that the third step of the process involves the touching of the sphere by a person. The person serves the role of the ground. If compared to the induction charging of a two-sphere system, the person has simply replaced the second sphere (Sphere B). Electrons within the sphere are repelled by the negative balloon and make an effort to distance themselves from it in order to minimize the repulsive affects. (This distance factor will be discussed in great detail in Lesson 3). While these electrons crowd to the right side of the sphere to distance themselves from the negatively charged balloon, they encounter another problem. In human terms, it could be said that the excess electrons on the right side of the sphere not only find the balloon to be repulsive, they also find each other to be repulsive. They simply need more space to distance themselves from the balloon as well as from each other. Quite regrettably for these electrons, they have run out of real estate; they cannot go further than the boundary of the sphere. Too many electrons in the same neighborhood is not a good thing. And when the hand comes nearby, these negative electrons see opportunity to find more real estate - a vast body of a human being into which they can roam and subsequently distance themselves even further from each other. It is in this sense, that the hand and the body to which it is attached (assuming of course that the hand is attached to a body) serve as a ground. A ground is simply a large object that serves as an almost infinite source of electrons or sink for electrons. A ground contains such vast space that it is the ideal object to either receive electrons or supply electrons to whatever object needs to get rid of them or receive them.
The second thing to note about the induction charging process shown above is that the sphere acquires a charge opposite the balloon. This will always be the observed case. If a negatively charged object is used to charge a neutral object by induction, then the neutral object will acquire a positive charge. And if a positively charged object is used to charge a neutral object by induction, then the neutral object will acquire a negative charge. If you understand the induction charging process, you can see why this would always be the case. The charged object that is brought near will always repel like charges and attract opposite charges. Either way, the object being charged acquires a charge that is opposite the charge of the object used to induce the charge. To further illustrate this, the diagram below shows how a positively charged balloon will charge a sphere negatively by induction.
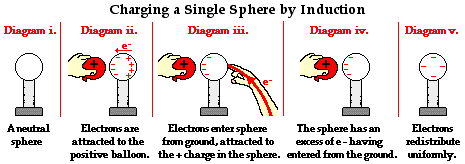
The Electrophorus
A commonly used lab activity that demonstrates the induction charging method is the Electrophorus Lab. In this lab, a flat plate of foam is rubbed with animal fur in order to impart a negative charge to the foam. Electrons are transferred from the animal fur to the more electron-loving foam (Diagram i.). An aluminum pie plate is taped to a Styrofoam cup; the aluminum is a conductor and the Styrofoam serves as an insulating handle. As the aluminum plate is brought near, electrons within the aluminum are repelled by the negatively charged foam plate. There is a mass migration of electrons to the rim of the aluminum pie plate. At this point, the aluminum pie plate is polarized, with the negative charge located along the upper rim farthest from the foam plate (Diagram ii.). The rim of the plate is then touched, providing a pathway from the aluminum plate to the ground. Electrons along the rim are not only repelled by the negative foam plate, they are also repelled by each other. So once touched, there is a mass migration of electrons from the rim to the person touching the rim (Diagram iii.). Being of much greater size than the aluminum pie plate, the person provides more space for the mutually repulsive electrons. The moment that electrons depart from the aluminum plate, the aluminum can be considered a charged object. Having lost electrons, the aluminum possesses more protons than electrons and is therefore positively charged. Once the foam plate is removed, the excess positive charge becomes distributed about the surface of the aluminum plate in order to minimize the overall repulsive forces between them (Diagram iv.).
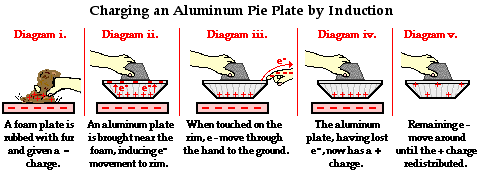
The Electrophorus Lab further illustrates that when charging a neutral object by induction, the charge imparted to the object is opposite that of the object used to induce the charge. In this case, the foam plate was negatively charged and the aluminum plate became positively charged. The lab also illustrates that there is never a transfer of electrons between the foam plate and the aluminum plate. The aluminum plate becomes charged by a transfer of electrons to the ground. Finally, one might note that the role of the charged object in induction charging is to simply polarize the object being charged. This polarization occurs as the negative foam plate repels electrons from the near side, inducing them to move to the opposite side of the aluminum plate. The presence of the positive charge on the bottom of the aluminum plate is the result of the departure of electrons from that location. Protons did not move downwards through the aluminum. The protons were always there from the beginning; it's just that they have lost their electron partners. Protons are fixed in place and incapable of moving in any electrostatic experiment.
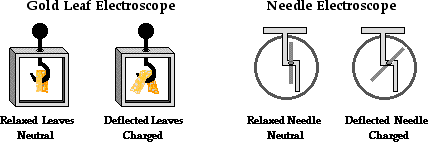
The Electroscope
Another common lab experience that illustrates the induction charging method is the Electroscope Lab. In the Electroscope Lab, a positively charged object such as an aluminum pie plate is used to charge an electroscope by induction. An electroscope is a device that is capable of detecting the presence of a charged object. It is often used in electrostatic experiments and demonstrations in order to test for charge and to deduce the type of charge present on an object. There are all kinds of varieties and brands of electroscope from the gold leaf electroscope to the needle electroscope.
While there are different types of electroscopes, the basic operation of each is the same. The electroscope typically consists of a conducting plate or knob, a conducting base and either a pair of conducting leaves or a conducting needle. Since the operating parts of an electroscope are all conducting, electrons are capable of moving from the plate or knob on the top of the electroscope to the needle or leaves in the bottom of the electroscope. Objects are typically touched to or held nearby the plate or knob, thus inducing the movement of electrons into the needle or the leaves (or from the needle/leaves to the plate/knob). The gold leaves or needle of the electroscope are the only mobile parts. Once an excess of electrons (or a deficiency of electrons) is present in the needle or the gold leaves, there will be a repulsive affect between like charges causing the leaves to repel each other or the needle to be repelled by the base that it rests upon. Whenever this movement of the leaves/needle is observed, one can deduce that an excess of charge - either positive or negative - is present there. It is important to note that the movement of the leaves and needle never directly indicate the type of charge on the electroscope; it only indicates that the electroscope is detecting a charge.
Suppose a needle electroscope is used to demonstrate induction charging. An aluminum pie plate is first charged positively by the process of induction (see discussion above). The aluminum plate is then held above the plate of the electroscope. Since the aluminum pie plate is not touched to the electroscope, the charge on the aluminum plate is NOT conducted to the electroscope. Nonetheless, the aluminum pie plate does have an affect upon the electrons in the electroscope. The pie plate induces electrons within the electroscope to move. Since opposites attract, a countless number of negatively charged electrons are drawn upwards towards the top of the electroscope. Having lost numerous electrons, the bottom of the electroscope has a temporarily induced positive charge. Having gained electrons, the top of the electroscope has a temporarily induced negative charge (Diagram ii. below). At this point the electroscope is polarized; however, the overall charge of the electroscope is neutral. The charging step then occurs as the bottom of the electroscope is touched to the ground. Upon touching the bottom of the electroscope, electrons enter the electroscope from the ground. One explanation of their entry is that they are drawn into the bottom of the electroscope by the presence of the positive charge at the bottom of the electroscope. Since opposites attract, electrons are drawn towards the bottom of the electroscope (Diagram iii.). As electrons enter, the needle of the electroscope is observed to return to the neutral position. This needle movement is the result of negative electrons neutralizing the previously positively charged needle at the bottom of the electroscope. At this point, the electroscope has an overall negative charge. The needle does not indicate this charge because the excess of electrons is still concentrated in the top plate of the electroscope; they are attracted to the positively charged aluminum pie plate that is held above the electroscope (Diagram iv.). Once the aluminum pie plate is pulled away, the excess of electrons in the electroscope redistribute themselves about the conducting parts of the electroscope. As they do, numerous excess electrons enter the needle and the base upon which the needle rests. The presence of excess negative charged in the needle and the base causes the needle to deflect, indicating that the electroscope has been charged (Diagram v.).
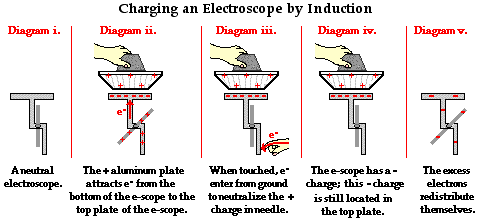
The above discussion provides one more illustration of the fundamental principles regarding induction charging. These fundamental principles have been illustrated in each example of induction charging discussed on this page. The principles are:
The charged object is never touched to the object being charged by induction.
The charged object does not transfer electrons to or receive electrons from the object being charged.
The charged object serves to polarize the object being charged.
The object being charged is touched by a ground; electrons are transferred between the ground and the object being charged (either into the object or out of it).
The object being charged ultimately receives a charge that is opposite that of the charged object that is used to polarize it.
Charging by Conduction
Charging by conduction involves the contact of a charged object to a neutral object. Suppose that a positively charged aluminum plate is touched to a neutral metal sphere. The neutral metal sphere becomes charged as the result of being contacted by the charged aluminum plate. Or suppose that a negatively charged metal sphere is touched to the top plate of a neutral needle electroscope. The neutral electroscope becomes charged as the result of being contacted by the metal sphere. And finally, suppose that an uncharged physics student stands on an insulating platform and touches a negatively charged Van de Graaff generator. The neutral physics student becomes charged as the result of contact with the Van de Graaff generator. Each of these examples involves contact between a charged object and a neutral object. In contrast to induction, where the charged object is brought near but never contacted to the object being charged, conduction charging involves making the physical connection of the charged object to the neutral object. Because charging by conduction involves contact, it is often called charging by contact.
Charging by Conduction Using a Negatively Charged Object
To explain the process of charging by contact, we will first consider the case of using a negatively charged metal sphere to charge a neutral needle electroscope. Understanding the process demands that you understand that like charges repel and have an intense desire to reduce their repulsions by spreading about as far as possible. A negatively charged metal sphere has an excess of electrons; those electrons find each other repulsive and distance themselves from each other as far as possible. The perimeter the sphere is the extreme to which they can go. If there was ever a conducting pathway to a more spacious piece of real estate, one could be sure that the electrons would be on that pathway to the greener grass beyond. In human terms, electrons living in the same home despise each other and are always seeking a home of their own or at least a home with more rooms.
Given this understanding of electron-electron repulsions, it is not difficult to predict what excess electrons on the metal sphere would be inclined to do if the sphere were touched to the neutral electroscope. Once the contact of the sphere to the electroscope is made, a countless number of excess electrons from the sphere move onto the electroscope and spread about the sphere-electroscope system. In general, the object that offers the most space in which to "hang out" will be the object that houses the greatest number of excess electrons. When the process of charging by conduction is complete, the electroscope acquires an excess negative charge due to the movement of electrons onto it from the metal sphere. The metal sphere is still charged negatively, only it has less excess negative charge than it had prior to the conduction charging process.
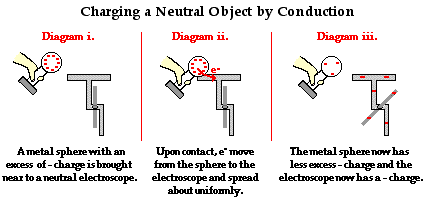
Charging by Conduction Using a Positively Charged Object
The previous example of charging by conduction involved touching a negatively charged object to a neutral object. Upon contact, electrons moved from the negatively charged object onto the neutral object. When finished, both objects were negatively charged. But what happens if a positively charged object is touched to a neutral object? To investigate this question, we consider the case of a positively charged aluminum plate being used to charge a neutral metal sphere by the process of conduction.
The diagram below depicts the use of a positively charged aluminum plate being touched to a neutral metal sphere. A positively charged aluminum plate has an excess of protons. When looked at from an electron perspective, a positively charged aluminum plate has a shortage of electrons. In human terms, we could say that each excess proton is rather discontented. It is not satisfied until it has found a negatively charged electron with which to co-habitate. However, since a proton is tightly bound in the nucleus of an atom, it is incapable of leaving an atom in search of that longed-for electron. It can however attract a mobile electron towards itself. And if a conducting pathway is made between a collection of electrons and an excess proton, one can be certain that there is likely an electron that would be willing to take the pathway. So when the positively charged aluminum plate is touched to the neutral metal sphere, countless electrons on the metal sphere migrate towards the aluminum plate. There is a mass migration of electrons until the positive charge on the aluminum plate-metal sphere system becomes redistributed. Having lost electrons to the positively charged aluminum plate, there is a shortage of electrons on the sphere and an overall positive charge. The aluminum plate is still charged positively; only it now has less excess positive charge than it had before the charging process began.
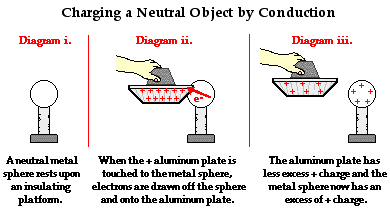
The above explanation might raise a rather difficult question: Why would an electron on the previously neutral metal sphere desire to move off the metal sphere in the first place? The metal sphere is neutral; every electron on it must be satisfied since there is a corresponding proton present. What would possibly induce an electron to go through the effort of migrating to a different territory in order to have what it already has?
The best means of answering this question requires an understanding of the concept of electric potential. But since that concept does not arise until the next unit of The Physics Classroom, a different approach to an answer will be taken. It ends up that electrons and protons are not as independent and individualized as we might think. From a human perspective, electrons and protons can't be thought of as independent citizens in a free enterprise system of government. Electrons and protons don't actually do what is best for themselves, but must be more social-minded. They must act like citizens of a state where the rule of law is to behave in a manner such that the overall repulsive affects within the society at large are reduced and the overall attractive affects are maximized. Electrons and protons will be motivated not by what is good for them, but rather by what is good for the country. And in this sense, a country's boundary extends to the perimeter of the conductor material that an excess electron is within. And in this case, an electron in the metal sphere is part of a country that extends beyond the sphere itself and includes the entire aluminum plate. So by moving from the metal sphere to the aluminum plate, an electron is able to reduce the total amount of repulsive affects within that country. It serves to spread the excess positive charge over a greater surface area, thus reducing the total amount of repulsive forces between excess protons.
Law of Conservation of Charge
In each of the other methods of charging discussed in Lesson 2 - charging by friction and charging by induction - the law of conservation of charge was illustrated. The law of conservation of charge states that charge is always conserved. When all objects involved are considered prior to and after a given process, we notice that the total amount of charge among the objects is the same before the process starts as it is after the process ends. The same conservation law is observed during the charging by conduction process. If a negatively charged metal sphere is used to charge a neutral electroscope, the overall charge before the process begins is the same as the overall charge when the process ends. So if before the charging process begins, the metal sphere has 1000 units of negative charge and the electroscope is neutral, the overall charge of the two objects in the system is -1000 units. Perhaps during the charging process, 600 units of negative charge moved from the metal sphere to the electroscope. When the process is complete, the electroscope would have 600 units of negative charge and the metal sphere would have 400 units of negative charge (the original 1000 units minus the 600 units it transferred to the electroscope). The overall charge of the two objects in the system is still -1000 units. The overall charge before the process began is the same as the overall charge when the process is completed. Charge is neither created nor destroyed; it is simply transferred from one object to another object in the form of electrons.
Conduction Charging Requires a Conductor
In all the above examples, the charging by conduction process involved the touching of two conductors. Does contact charging have to occur through the contact of two conductors? Can an insulator conduct a charge to another object upon touching? And can an insulator be charged by conduction? A complete discussion of these questions can get messy and quite often leads to a splitting of hairs over the definition of conduction and the distinction between conductors and 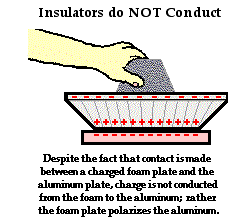 insulators. The belief is taken here that only a conductor can conduct charge to another conductor. The process of noticeably charging an object by contact involves the two contacting objects momentarily sharing the net excess charge. The excess charge is simply given a larger area over which to spread in order to reduce the total amount of repulsive forces between them. This process demands that the objects be conductors in order for electrons to move about and redistribute themselves. An insulator hinders such a movement of electrons between touching objects and about the surfaces of the objects. This is observed if an aluminum pie plate is placed upon a charged foam plate. When the neutral aluminum plate is placed upon the charged foam plate, the foam plate does not conduct its charge to the aluminum. Despite the fact that the two surfaces were in contact, charging by contact or conduction did not occur. (Or at least whatever charge transfer might have occurred was not noticeable by the customary means of using an electroscope, using a charge testing bulb or testing for its repulsion with a like-charged object.)
insulators. The belief is taken here that only a conductor can conduct charge to another conductor. The process of noticeably charging an object by contact involves the two contacting objects momentarily sharing the net excess charge. The excess charge is simply given a larger area over which to spread in order to reduce the total amount of repulsive forces between them. This process demands that the objects be conductors in order for electrons to move about and redistribute themselves. An insulator hinders such a movement of electrons between touching objects and about the surfaces of the objects. This is observed if an aluminum pie plate is placed upon a charged foam plate. When the neutral aluminum plate is placed upon the charged foam plate, the foam plate does not conduct its charge to the aluminum. Despite the fact that the two surfaces were in contact, charging by contact or conduction did not occur. (Or at least whatever charge transfer might have occurred was not noticeable by the customary means of using an electroscope, using a charge testing bulb or testing for its repulsion with a like-charged object.)
Many might quickly suggest that they have used a charged insulator to charge a neutral electroscope (or some other object) by contact. In fact, a negatively charged plastic golf tube can used to charge an electroscope. The plastic tube is touched to the top plate of the electroscope. On most occasions, the plastic tube is even rubbed or rolled across the plate of the electroscope? Wouldn't this be regarded as charging by conduction? No. Not really. In this case, it is more than  likely that the charging occurred by some process other than conduction. There was not a sharing of charge between the plastic tube and the metal parts of the electroscope. Of course, once some excess charge is acquired by the electroscope, that excess charge distributes itself about the surface of the electroscope. Yet the charge is not uniformly shared between the two objects. The protons and electrons within both the plastic golf tube and the electroscope are not acting together to share excess charge and reduce the total amount of repulsive forces.
likely that the charging occurred by some process other than conduction. There was not a sharing of charge between the plastic tube and the metal parts of the electroscope. Of course, once some excess charge is acquired by the electroscope, that excess charge distributes itself about the surface of the electroscope. Yet the charge is not uniformly shared between the two objects. The protons and electrons within both the plastic golf tube and the electroscope are not acting together to share excess charge and reduce the total amount of repulsive forces.
The charging of an electroscope by contact with a negatively charged golf tube (or any charged insulating object) would best be described as charging by lightning. Rather than being a process in which the two objects act together to share the excess charge, the process could best be described as the successful effort of electrons to burst through the space (air) between objects. The presence of a negatively charged plastic tube is capable of ionizing the air surrounding the tube and allowing excess electrons on the plastic tube to be conducted through the air to the electroscope. This transfer of charge can happen with or without touching. In fact, on a dry winter day the process of charging the metal electroscope with the charged insulator often occurs while the insulator is some distance away. The dry air is more easily ionized and a greater quantity of electrons is capable of bursting through the space between the two objects. On such occasions, a crackling sound is often heard and a flash of light is seen if the room is darkened. This phenomenon, occurring from several centimeters away, certainly does not fit the description of contact charging.
A charged insulating object is certainly capable of transferring its charge to another object. The result of the charge transfer will be the same as the result of charging by conduction. Both objects will have the same type of charge and the flow of electrons is in the same direction. However, the process and the underlying explanations are considerably different. In the case of charging an object with a charged insulator, the contact is not essential. Contacting the object simply reduces the spatial separation between touching atoms and allows charge to arc and spark its way between objects. Rubbing or rolling the insulating object across the conductor's surface facilitates the charging process by bringing a greater number of atoms on the insulator in close proximity to the conductor that is receiving the charge. The two materials do not make any effort to share charge nor to act as a single object (with a uniform electric potential) in an effort to reduce repulsive affects.
Is this distinction between charging by conduction and charging by lightning a splitting of hairs? Perhaps. For certain, each process involves a transfer of charge from one object to another object, yielding the same result - two like-charged object. Yet, distinguishing between the two forms of charging is more consistent with the customary view that insulators are not conductors of charge. It also serves to explain why some insulators clearly do not always transfer their charge upon contact. This phenomenon of charging by lightning will be revisited in Lesson 4 during a discussion of electric fields and lightning discharges.
Grounding
The previous three sections of Lesson 2 discussed the three common methods of charging - charging by friction, charging by induction, and charging by conduction. A discussion of charging would not be complete without a discussion of uncharging. Objects with an excess of charge - either positive or negative - can have this charge removed by a process known as grounding. Grounding is the process of removing the excess charge on an object by means of the transfer of electrons between it and another object of substantial size. When a charged object is grounded, the excess charge is balanced by the transfer of electrons between the charged object and a ground. A ground is simply an object that serves as a seemingly infinite reservoir of electrons; the ground is capable of transferring electrons to or receiving electrons from a charged object in order to neutralize that object. In this last section of Lesson 2, the process of grounding will be discussed.
Grounding a Negatively Charged Object
To begin our discussion of grounding, we will consider the grounding of a negatively charged electroscope. Any negatively charged object has an excess of electrons. If it is to have its charge 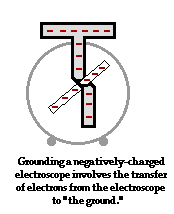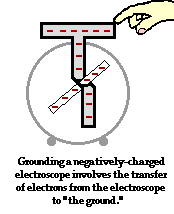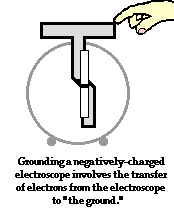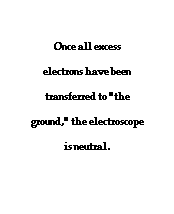 removed, then it will have to lose its excess electrons. Once the excess electrons are removed from the object, there will be equal numbers of protons and electrons within the object and it will have a balance of charge. To remove the excess of electrons from a negatively charged electroscope, the electroscope will have to be connected by a conducting pathway to another object that is capable of receiving those electrons. The other object is the ground. In typical electrostatic experiments and demonstrations, this is simply done by touching the electroscope with one's hand. Upon contact, the excess electrons leave the electroscope and enter the person who touches it. These excess electrons subsequently spread about the surface of the person.
removed, then it will have to lose its excess electrons. Once the excess electrons are removed from the object, there will be equal numbers of protons and electrons within the object and it will have a balance of charge. To remove the excess of electrons from a negatively charged electroscope, the electroscope will have to be connected by a conducting pathway to another object that is capable of receiving those electrons. The other object is the ground. In typical electrostatic experiments and demonstrations, this is simply done by touching the electroscope with one's hand. Upon contact, the excess electrons leave the electroscope and enter the person who touches it. These excess electrons subsequently spread about the surface of the person.
This process of grounding works because excess electrons find each other repulsive. As is always the case, repulsive affects between like-charged electrons forces them to look for a means of spatially separating themselves from each other. This spatial separation is achieved by moving to a larger object that allows a greater surface area over which to spread. Because of the relative size of a person compared to a typical electroscope, the excess electrons (nearly all of them) are capable of reducing the repulsive forces by moving into the person (i.e., the ground). Like contact charging discussed earlier, grounding is simply another example of charge sharing between two objects. The extent to which an object is willing to share excess charge is proportional to its size. So an effective ground is simply an object with significant enough size to share the overwhelming majority of excess charge.
Grounding a Positively Charged Object
The previous discussion describes the grounding of a negatively charged electroscope. Electrons were transferred from the 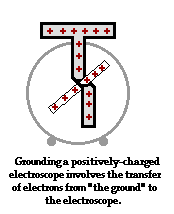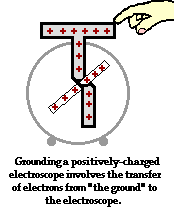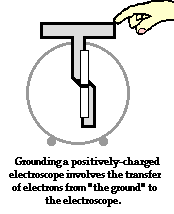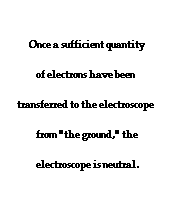 electroscope to the ground. But what if the electroscope is positively charged? How does electron transfer allow an object with an excess of protons to become neutralized? To explore these questions, we will consider the grounding of a positively charged electroscope. A positively charged electroscope must gain electrons in order to acquire an equal number of protons and electrons. By gaining electrons from the ground, the electroscope will have a balance of charge and therefore be neutral. Thus, the grounding of a positively charged electroscope involves the transfer of electrons from the ground into the electroscope. This process works because excess positive charge on the electroscope attracts electrons from the ground (in this case, a person). While this may disrupt any balance of charge present on the person, the significantly larger size of the person allows for the excess charge to distance itself further from each other. As in the case of grounding a negatively charged electroscope, the grounding of a positively charged electroscope involves charge sharing. The excess positive charge is shared between the electroscope and the ground. And once again, the extent to which an object is willing to share excess charge is proportional to its size. The person is an effective ground because it has enough size to share the overwhelming majority of excess positive charge.
electroscope to the ground. But what if the electroscope is positively charged? How does electron transfer allow an object with an excess of protons to become neutralized? To explore these questions, we will consider the grounding of a positively charged electroscope. A positively charged electroscope must gain electrons in order to acquire an equal number of protons and electrons. By gaining electrons from the ground, the electroscope will have a balance of charge and therefore be neutral. Thus, the grounding of a positively charged electroscope involves the transfer of electrons from the ground into the electroscope. This process works because excess positive charge on the electroscope attracts electrons from the ground (in this case, a person). While this may disrupt any balance of charge present on the person, the significantly larger size of the person allows for the excess charge to distance itself further from each other. As in the case of grounding a negatively charged electroscope, the grounding of a positively charged electroscope involves charge sharing. The excess positive charge is shared between the electroscope and the ground. And once again, the extent to which an object is willing to share excess charge is proportional to its size. The person is an effective ground because it has enough size to share the overwhelming majority of excess positive charge.
The Need for a Conducting Pathway
Any object can be grounded provided that the charged atoms of that object have a conducting pathway between the atoms and the ground. A common lab activity involves taping two straws to a charged aluminum plate. One straw is covered with aluminum foil and the other straw is bare plastic. When the aluminum-covered straw is touched, the aluminum plate loses its charge. It is grounded by means of the movement of electrons from the ground to the aluminum plate. When the plastic straw is touched, grounding does not occur. The plastic serves as an insulator and prevents the flow of electrons from the ground to the aluminum plate. Grounding requires a conducting pathway between the ground and the object to be grounded. Electrons will travel along that pathway.
Physics - Electrostatics
Neutral v. Charged Objects
As discussed in a previous section of Lesson 1, atoms are the building blocks of matter. There are different types of atoms, known as elements. Atoms of each element are distinguished from each other by the number of protons that are present in their nucleus. An atom containing one proton is a hydrogen atom (H). An atom containing 6 protons is a carbon atom. And an atom containing 8 protons is an oxygen atom.
The number of electrons that surround the nucleus will determine whether or not an atom is electrically charged or electrically neutral. The amount of charge on a single proton is equal to the amount of charge possessed by a single electron. A proton and an electron have an equal amount but an opposite type of charge. Thus, if an atom contains equal numbers of protons and electrons, the atom is described as being electrically neutral. On the other hand, if an atom has an unequal number of protons and electrons, then the atom is electrically charged (and in fact, is then referred to as an ion rather than an atom). Any particle, whether an atom, molecule or ion, that contains less electrons than protons is said to be positively charged. Conversely, any particle that contains more electrons than protons is said to be negatively charged.
Charged versus Uncharged Particles | ||
Positively Charged | Negatively Charged | Uncharged |
Possesses more protons than electrons | Possesses more electrons than protons | Equal numbers of protons and electrons |
Charged Objects as an Imbalance of Protons and Electrons
In the previous section of Lesson 1, an atom was described as being a small and dense core of positively charged protons and neutral neutrons surrounded by shells of negatively charged electrons. The protons are tightly bound within the nucleus and not removable by ordinary measures. While the electrons are attracted to the protons of the nucleus, the addition of energy to an atom can persuade the electrons to leave an atom. Similarly, electrons within atoms of other materials can be persuaded to leave their own electron shells and become members of the electrons shells of other atoms of different materials. In short, electrons are migrants - constantly on the move and always ready to try out a new atomic environment.
 All objects are composed of these atoms. The electrons contained within the objects are prone to move or migrate to other objects. The process of an electron leaving one material object to reside (perhaps only temporarily) in another object is a common everyday occurrence. Even as you read the words of this web page, some electrons are likely moving through the monitor and adhering to your clothing (assuming that you are using this resource online) (and wearing clothes). If you were to walk across the carpeting towards the door of the room, electrons would likely be scuffed off the atoms of your shoes and moved onto the atoms of the carpet. And as clothes tumble in the dryer, it is highly likely that electrons on one piece of clothing will move from the atoms of the clothing onto the atoms of another piece of clothing. In general, for electrons to make a move from the atoms of one material to the atoms of another material, there must be an energy source, a motive, and a low-resistance pathway.
All objects are composed of these atoms. The electrons contained within the objects are prone to move or migrate to other objects. The process of an electron leaving one material object to reside (perhaps only temporarily) in another object is a common everyday occurrence. Even as you read the words of this web page, some electrons are likely moving through the monitor and adhering to your clothing (assuming that you are using this resource online) (and wearing clothes). If you were to walk across the carpeting towards the door of the room, electrons would likely be scuffed off the atoms of your shoes and moved onto the atoms of the carpet. And as clothes tumble in the dryer, it is highly likely that electrons on one piece of clothing will move from the atoms of the clothing onto the atoms of another piece of clothing. In general, for electrons to make a move from the atoms of one material to the atoms of another material, there must be an energy source, a motive, and a low-resistance pathway.
The cause and mechanisms by which this movement of electrons occurs will be the subject of Lesson 2. For now, it is sufficient to say that objects that are charged contain unequal numbers of protons and electrons. Charged objects have an imbalance of charge - either more negative electrons than positive protons or vice versa. And neutral objects have a balance of charge - equal numbers of protons and electrons. The principle stated earlier for atoms can be applied to objects. Objects with more electrons than protons are charged negatively; objects with fewer electrons than protons are charged positively.
In this discussion of electrically charged versus electrically neutral objects, the neutron has been neglected. Neutrons, being electrically neutral play no role in this unit. Their presence (or absence) will have no direct bearing upon whether an object is charged or uncharged. Their role in the atom is merely to provide stability to the nucleus, a subject not discussed in The Physics Classroom. When it comes to the drama of static electricity, electrons and protons become the main characters.
Charge as a Quantity
Like mass, the charge of an object is a measurable quantity. The charge possessed by an object is often expressed using the scientific unit known as the Coulomb. Just as mass is measured in grams or kilograms, charge is measured in units of Coulombs (abbreviated C). Because one Coulomb of charge is an abnormally large quantity of charge, the units of microCoulombs (µC) or nanoCoulombs (nC) are more commonly used as the unit of measurement of charge. To illustrate the magnitude of 1 Coulomb, an object would need an excess of 6.25 x 1018 electrons to have a total charge of -1 C. And of course an object with a shortage of 6.25 x 1018 electrons would have a total charge of +1 C.
The charge on a single electron is -1.6 x 10 -19 Coulomb. The charge on a single proton is +1.6 x 10 -19 Coulomb. The quantity of charge on an object reflects the amount of imbalance between electrons and protons on that object. Thus, to determine the total charge of a positively charged object (an object with an excess of protons), one must subtract the total number of electrons from the total number of protons. This operation yields the number of excess protons. Since a single proton contributes a charge of +1.6 x 10 -19 Coulomb to the overall charge of an atom, the total charge can be computed by multiplying the number of excess protons by +1.6 x 10 -19 Coulomb. A similar process is used to determine the total charge of a negatively charged object (an object with an excess of electrons), except that the number of protons is first subtracted from the number of electrons.
This principle is illustrated in the following table.
Object | # of Excess Protons/Electrons | Quantity and Kind of Charge (Q) on Object in Coulombs (C) |
A | 1 x 106 excess electrons | -1.6 x 10-13 C |
B | 1 x 106 excess protons | +1.6 x 10-13 C |
C | 2 x 1010 excess electrons | -3.2 x 10-9 C |
D | 3.5 x 108 excess protons | +5.6 x 10-11 C |
E | 4.67 x 1010 excess electrons | -7.5 x 10-9 C |
In conclusion, an electrically neutral object is an object that has a balance of protons and electrons. In contrast, a charged object has an imbalance of protons and electrons. Determining the quantity of charge on such an object involves a counting process; the total number of electrons and protons are compared to determine the difference between the number of protons and electrons. This difference is multiplied by 1.6 x 10 -19 Coulombs to determine the overall quantity of charge on the object. The type of charge (positive or negative) is determined by whether the protons or the electrons are in excess.
Charge Interactions
Suppose that you rubbed a balloon with a sample of animal fur such as a wool sweater or even your own hair. The balloon would likely become charged and its charge would exert a strange influence upon other objects in its vicinity. If some small bits of paper were placed upon a table and the balloon were brought near and held above the paper bits, then the presence of the charged balloon might create a sufficient attraction for the paper bits to raise them off the table. This influence - known as an electric force - occurs even when the charged balloon is held some distance away from the paper bits. The electric force is a non-contact force. Any charged object can exert this force upon other objects - both charged and uncharged objects. One goal of this unit of The Physics Classroom is to understand the nature of the electric force. In this part of Lesson 1, two simple and fundamental statements will be made and explained about the nature of the electric force.
Perhaps you have heard it said so many times that it sounds like a cliché.
Opposites attract. And likes repel.
These two fundamental principles of charge interactions will be used throughout the unit to explain the vast array of static electricity phenomena. As mentioned in the previous section of Lesson 1, there are two types of electrically charged objects - those that contain more protons than electrons and are said to be positively charged and those that contain less protons than electrons and are said to be negatively charged. These two types of electrical charges - positive and negative - are said to be opposite types of charge. And consistent with our fundamental principle of charge interaction, a positively charged object will attract a negatively charged object. Oppositely charged objects will exert an attractive influence upon each other. In contrast to the attractive force between two objects with opposite charges, two objects that are of like charge will repel each other. That is, a positively charged object will exert a repulsive force upon a second positively charged object. This repulsive force will push the two objects apart. Similarly, a negatively charged object will exert a repulsive force upon a second negatively charged object. Objects with like charge repel each other.

The Electric Force and Newton's Third Law
This electric force exerted between two oppositely charged objects or two like charged objects is a force in the same sense that friction, tension, gravity and air resistance are forces. And being a force, the same laws and principles that describe any force describe the electrical force. One of those laws was Newton's law of action-reaction (discussed in Unit 2 of The Physics Classroom). According to Newton's third law, a force is simply a mutual interaction between two objects that results in an  equal and opposite push or pull upon those objects. Let's apply Newton's third law to describe the interaction between Object A and Object B, both having positive charge.
equal and opposite push or pull upon those objects. Let's apply Newton's third law to describe the interaction between Object A and Object B, both having positive charge.
Object A exerts a rightward push upon Object B. Object B exerts a leftward push upon Object A. See diagram at right. These two pushing forces have equal magnitudes and are exerted in opposite directions of each other. Each object does its own pushing upon the other. The push upon Object B (by Object A) is directed away from Object A; and the push upon Object A (by Object B) is directed away from Object B. Because of the away from nature of the mutual interaction, the force is said to be repulsive.
 Now let's apply the same action-reaction principle to two oppositely charged objects - Object C (positive) and Object D (negative). See diagram at right. Object C exerts a leftward pull upon object D. Object D exerts a rightward pull upon Object C. Again, each object does its own pulling of the other. Just as before, these two forces have equal magnitudes and are exerted in opposite directions of each other. However in this instance, the direction of the force on Object D is towards Object C and the direction of the force on Object C is towards object D. Because of the towards each other nature of the mutual interaction, the force is described as being attractive.
Now let's apply the same action-reaction principle to two oppositely charged objects - Object C (positive) and Object D (negative). See diagram at right. Object C exerts a leftward pull upon object D. Object D exerts a rightward pull upon Object C. Again, each object does its own pulling of the other. Just as before, these two forces have equal magnitudes and are exerted in opposite directions of each other. However in this instance, the direction of the force on Object D is towards Object C and the direction of the force on Object C is towards object D. Because of the towards each other nature of the mutual interaction, the force is described as being attractive.
Interaction Between Charged and Neutral Objects
The interaction between two like-charged objects is repulsive. The interaction between two oppositely charged objects is attractive. What type of interaction is observed between a charged object and a neutral object? The answer is quite surprising to many students of physics. Any charged object - whether positively charged or negatively charged - will have an attractive interaction with a neutral object. Positively charged objects and neutral objects attract each other; and negatively charged objects and neutral objects attract each other.
 This third interaction between charged and neutral objects is often demonstrated by physics teachers or experienced by students in physics lab activities. For instance, if a charged balloon is held above neutral bits of paper, the force of attraction for the paper bits will be strong enough to overwhelm the downward force of gravity and raise the bits of paper off the table. If a charged plastic tube is held above some bits of paper, the tube will exert an attractive influence upon the paper to raise it off the table. And to the bewilderment of many, a charged rubber balloon can be attracted to a wooden cabinet with enough force that it sticks to the cabinet. Any charged object - plastic, rubber, or aluminum - will exert an attractive force upon a neutral object. And in accordance with Newton's law of action-reaction, the neutral object attracts the charged object.
This third interaction between charged and neutral objects is often demonstrated by physics teachers or experienced by students in physics lab activities. For instance, if a charged balloon is held above neutral bits of paper, the force of attraction for the paper bits will be strong enough to overwhelm the downward force of gravity and raise the bits of paper off the table. If a charged plastic tube is held above some bits of paper, the tube will exert an attractive influence upon the paper to raise it off the table. And to the bewilderment of many, a charged rubber balloon can be attracted to a wooden cabinet with enough force that it sticks to the cabinet. Any charged object - plastic, rubber, or aluminum - will exert an attractive force upon a neutral object. And in accordance with Newton's law of action-reaction, the neutral object attracts the charged object.
Flickr Physics Photo
A balloon is charged by rubbing it with hair. It is then brought near some bits of paper. The charged balloon attracts the paper bits, lifting them up off the table. This demonstrates the attraction between charged objects and neutral objects.
Repulsion versus Attraction
 Because charged objects interact with their surroundings, an observed interaction provides possible evidence that an object is charged. Suppose that you enter the physics classroom and observe two balloons suspended from the ceiling. Rather than hanging straight down vertically, the balloons are hanging at an angle, exhibiting a repulsive interaction as shown at the right. The only way that two objects can repel each other is if they are both charged with the same type of charge. Thus, the repulsion of the balloons provides conclusive evidence that both balloons are charged and charged with the same type of charge. One could not conclude that the balloons are both positively charged or both negatively charged. Additional information or further testing would be required to make a conclusion about the type of excess charge present upon the balloons. Nonetheless, one can be convinced that both balloons possess an excess charge - either positive or negative.
Because charged objects interact with their surroundings, an observed interaction provides possible evidence that an object is charged. Suppose that you enter the physics classroom and observe two balloons suspended from the ceiling. Rather than hanging straight down vertically, the balloons are hanging at an angle, exhibiting a repulsive interaction as shown at the right. The only way that two objects can repel each other is if they are both charged with the same type of charge. Thus, the repulsion of the balloons provides conclusive evidence that both balloons are charged and charged with the same type of charge. One could not conclude that the balloons are both positively charged or both negatively charged. Additional information or further testing would be required to make a conclusion about the type of excess charge present upon the balloons. Nonetheless, one can be convinced that both balloons possess an excess charge - either positive or negative.
 Now let's contrast the observation of repulsion with that of attraction. Suppose that you now enter the physics classroom and observe two balloons suspended from the ceiling and exhibiting an attractive interaction as shown at the right. There are two underlying reasons for two objects attracting each other. One balloon could be neutral and the other balloon charged or both balloons could be charged with the opposite type of charge. Thus, your only conclusion could be that at least one of the balloons is charged. The other balloon is either neutral or charged with the opposite type of charge. You cannot draw a conclusion about which one of the balloons is charged or what type of charge (positive or negative) the charged balloon possesses. Additional information or further testing would be required to make these conclusions. For example, if you could take each balloon and individually bring them near some neutral bits of paper, you could test to see if each individual balloon is charged or neutral. If a balloon were charged, then it would exhibit an attractive interaction with the neutral paper bits. On the other hand, an uncharged balloon would not interact at all with neutral paper bits.
Now let's contrast the observation of repulsion with that of attraction. Suppose that you now enter the physics classroom and observe two balloons suspended from the ceiling and exhibiting an attractive interaction as shown at the right. There are two underlying reasons for two objects attracting each other. One balloon could be neutral and the other balloon charged or both balloons could be charged with the opposite type of charge. Thus, your only conclusion could be that at least one of the balloons is charged. The other balloon is either neutral or charged with the opposite type of charge. You cannot draw a conclusion about which one of the balloons is charged or what type of charge (positive or negative) the charged balloon possesses. Additional information or further testing would be required to make these conclusions. For example, if you could take each balloon and individually bring them near some neutral bits of paper, you could test to see if each individual balloon is charged or neutral. If a balloon were charged, then it would exhibit an attractive interaction with the neutral paper bits. On the other hand, an uncharged balloon would not interact at all with neutral paper bits.
The above thought experiments illustrate the conclusive nature of a repulsive interaction. When objects repel each other, one can be certain that both objects are charged. On the other had, the observation of an attractive interaction leads to limited conclusions. At best, one can conclude that at least one of the objects is charged.
Conductors and Insulators
The behavior of an object that has been charged is dependent upon whether the object is made of a conductive or a nonconductive material. Conductors are materials that permit electrons to flow freely from particle to particle. An object made of a conducting material will permit charge to be transferred across the entire surface of the object. If charge is transferred to the object at a given location, that charge is quickly distributed across the entire surface of the object. The distribution of charge is the result of electron movement. Since conductors allow for electrons to be transported from particle to particle, a charged object will always distribute its charge until the overall repulsive forces between excess electrons is minimized. If a charged conductor is touched to another object, the conductor can even transfer its charge to that object. The transfer of charge between objects occurs more readily if the second object is made of a conducting material. Conductors allow for charge transfer through the free movement of electrons.

In contrast to conductors, insulators are materials that impede the free flow of electrons from atom to atom and molecule to molecule. If charge is transferred to an insulator at a given location, the excess charge will remain at the initial location of charging. The particles of the insulator do not permit the free flow of electrons; subsequently charge is seldom distributed evenly across the surface of an insulator.
While insulators are not useful for transferring charge, they do serve a critical role in electrostatic experiments and demonstrations. Conductive objects are often mounted upon insulating objects. This arrangement of a conductor on top of an insulator prevents charge from being transferred from the conductive object to its surroundings. This arrangement also allows for a student (or teacher) to manipulate a conducting object without touching it. The insulator serves as a handle for moving the conductor around on top of a lab table. If charging experiments are performed with aluminum pop cans, then the cans should be mounted on top of Styrofoam cups. The cups serve as insulators, preventing the pop cans from discharging their charge. The cups also serve as handles when it becomes necessary to move the cans around on the table.

Examples of Conductors and Insulators
Examples of conductors include metals, aqueous solutions of salts (i.e., ionic compounds dissolved in water), graphite, and the human body. Examples of insulators include plastics, Styrofoam, paper, rubber, glass and dry air. The division of materials into the categories of conductors and insulators is a somewhat artificial division. It is more appropriate to think of materials as being placed somewhere along a continuum. Those materials that are super conductive (known as superconductors) would be placed at on end and the least conductive materials (best insulators) would be placed at the other end. Metals would be placed near the most conductive end and glass would be placed on the opposite end of the continuum. The conductivity of a metal might be as much as a million trillion times greater than that of glass.

Along the continuum of conductors and insulators, one might find the human body somewhere towards the conducting side of the middle. When  the body acquires a static charge it has a tendency to distribute that charge throughout the surface of the body. Given the size of the human body, relative to the size of typical objects used in electrostatic experiments, it would require an abnormally large quantity of excess charge before its effect is noticeable. The effects of excess charge on the body are often demonstrated using a Van de Graaff generator. When a student places their hand upon the static ball, excess charge from the ball is shared with the human body. Being a conductor, the excess charge could flow to the human body and spread throughout the surface of the body, even onto strands of hair. As the individual strands of hair become charged, they begin to repel each other. Looking to distance themselves from their like-charged neighbors, the strands of hair begin to rise upward and outward - a truly hair-raising experience.
the body acquires a static charge it has a tendency to distribute that charge throughout the surface of the body. Given the size of the human body, relative to the size of typical objects used in electrostatic experiments, it would require an abnormally large quantity of excess charge before its effect is noticeable. The effects of excess charge on the body are often demonstrated using a Van de Graaff generator. When a student places their hand upon the static ball, excess charge from the ball is shared with the human body. Being a conductor, the excess charge could flow to the human body and spread throughout the surface of the body, even onto strands of hair. As the individual strands of hair become charged, they begin to repel each other. Looking to distance themselves from their like-charged neighbors, the strands of hair begin to rise upward and outward - a truly hair-raising experience.
Many are familiar with the impact that humidity can have upon static charge buildups. You have likely noticed that bad hair days, doorknob shocks and static clothing are most common during winter months. Winter months tend to be the driest months of the year with humidity levels in the air dropping to lower values. Water has a tendency to gradually remove excess charge from objects. When the humidity is high, a person acquiring an excess charge will tend to lose that charge to water molecules in the surrounding air. On the other hand, dry air conditions are more conducive to the buildup of static charge and more frequent electric shocks. Since humidity levels tend to vary from day to day and season to season, it is expected that electrical effects (and even the success of electrostatic demonstrations) can vary from day to day.
Distribution of Charge via Electron Movement
Predicting the direction that electrons would move within a conducting material is a simple application of the two fundamental rules of charge interaction. Opposites attract and likes repel. Suppose that some method is used to impart a negative charge to an object at a given location. At the location where the charge is imparted, there is an excess of electrons. That is, the multitude of atoms in that region possess more electrons than protons. Of course, there are a number of electrons that could be thought of as being quite contented since there is an accompanying positively charged proton to satisfy their attraction for an opposite. However, the so-called excess electrons have a repulsive response to each other and would prefer more space. Electrons, like human beings, wish to manipulate their surroundings in an effort to reduce repulsive effects. Since these excess electrons are present in a conductor, there is little hindrance to their ability to migrate to other parts of the object. And that is exactly what they do. In an effort to reduce the overall repulsive effects within the object, there is a mass migration of excess electrons throughout the entire surface of the object. Excess electrons migrate to distance themselves from their repulsive neighbors. In this sense, it is said that excess negative charge distributes itself throughout the surface of the conductor.
But what happens if the conductor acquires an excess of positive charge? What if electrons are removed from a conductor at a given location, giving the object an overall positive charge? If protons cannot move, then how can the excess of positive charge distribute itself across the surface of the material? While the answers to these questions are not as obvious, it still involves a rather simple explanation that once again relies on the two fundamental rules of charge interaction. Opposites attract and likes repel. Suppose that a conducting metal sphere is charged on its left side and imparted an excess of positive charge. (Of course, this requires that electrons be removed from the object at the location of charging.) A multitude of atoms in the region where the charging occurs have lost one or more electrons and have an excess of protons. The imbalance of charge within these atoms creates effects that can be thought of as disturbing the balance of charge within the entire object. The presence of these excess protons in a given location draws electrons from other atoms. Electrons in other parts of the object can be thought of as being quite contented with the balance of charge that they are experiencing. Yet there will always be some electrons that will feel the attraction for the excess protons some distance away. In human terms, we might say these electrons are drawn by curiosity or by the belief that the grass is greener on the other side of the fence. In the language of electrostatics, we simply assert that opposites attract - the excess protons and both the neighboring and distant electrons attract each other. The protons cannot do anything about this attraction since they are bound within the nucleus of their own atoms. Yet, electrons are loosely bound within atoms; and being present in a conductor, they are free to move. These electrons make the move for the excess protons, leaving their own atoms with their own excess of positive charge. This electron migration happens across the entire surface of the object, until the overall sum of repulsive effects between electrons across the whole surface of the object are minimized.
Polarization
Inducing the Movement of Charge
As discussed previously, an atom consists of positively charged protons and negatively charged electrons. The protons are in the nucleus of the atom, tightly bound and incapable of movement. The electrons are located in the vast regions of space surrounding the nucleus, known as the electron shells or the electron clouds. Relative to the protons of the nucleus, these electrons are loosely bound. In conducting objects, they are so loosely bound that they may be induced into moving from one portion of the object to another portion of the object. To get an electron in a conducting object to get up and go, all that must be done is to place a charged object nearby the conducting object.
To illustrate this induced movement of electrons, we will consider an aluminum pop can that is taped to a Styrofoam cup. The Styrofoam cup serves as both an insulating stand and a handle. A rubber balloon is charged negatively, perhaps by rubbing it against animal fur. If the negatively charged balloon is brought near the aluminum pop can, the electrons within the pop can will experience a repulsive force. The repulsion will be greatest for those electrons that are nearest the negatively charged balloon. Many of these electrons will be induced into moving away from the repulsive balloon. Being present within a conducting material, the electrons are free to move from atom to atom. As such, there is a mass migration of electrons from the balloon's side of the aluminum can towards the opposite side of the can. This electron movement leaves atoms on the balloon's side of the can with a shortage of electrons; they become positively charged. And the atoms on the side opposite of the can have an excess of electrons; they become negatively charged. The two sides of the aluminum pop can have opposite charges. Overall the can is electrically neutral; it's just that the positive and negative charge has been separated from each other. We say that the charge in the can has been polarized.

In general terms, polarization means to separate into opposites. In the political world, we often observe that a collection of people becomes polarized over some issue. For instance, we might say that the United States has become polarized over the issue of the death penalty. That is, the citizens of the United States have been separated into opposites - those who are for the death penalty and those who are against the death penalty. In the context of electricity, polarization is the process of separating opposite charges within an object. The positive charge becomes separated from the negative charge. By inducing the movement of electrons within an object, one side of the object is left with an excess of positive charge and the other side of the object is left with an excess of negative charge. Charge becomes separated into opposites.
The polarization process always involves the use of a charged object to induce electron movement or electron rearrangement. In the above diagram and accompanying discussion, electrons within a conducting object were induced into moving from the left side of the conducting can to the right side of the can. Being a conductor, electrons were capable of moving from atom to atom across the entire surface of the conductor. But what if the object being polarized is an insulator? Electrons are not free to move across the surface of an insulator. How can an insulator such as a wooden wall be polarized?
How Can an Insulator be Polarized?
Polarization can occur within insulators, but the process occurs in a different manner than it does within a conductor. In a conducting object, electrons are induced into movement across the surface of the conductor from one side of the object to the opposite side. In an insulator, electrons merely redistribute themselves within the atom or molecules nearest the outer surface of the object. To understand the electron redistribution process, it is important to take another brief excursion into the world of atoms, molecules and chemical bonds.
The electrons surrounding the nucleus of an atom are believed to be located in regions of space with specific shapes and sizes. The actual size and shape of these regions is determined by the high-powered mathematical equations common to Quantum Mechanics. Rather than being located a specific distance from the nucleus in a fixed orbit, the electrons are simply thought of as being located in regions often referred to as electron clouds. At any given moment, the electron is likely to be found at some location within the cloud. The electron clouds have varying density; the density of the cloud is considered to be greatest in the portion of the cloud where the electron has the greatest probability of being found at any given moment. And conversely, the electron cloud density is least in the regions where the electron is least likely to be found. In addition to having varying density, these electron clouds are also highly distortable. The presence of neighboring atoms with high electron affinity can distort the electron clouds around atoms. Rather than being located symmetrically about the positive nucleus, the cloud becomes asymmetrically shaped. As such, there is a polarization of the atom as the centers of positive and negative charge are no longer located in the same location. The atom is still a neutral atom; it has just become polarized.

The discussion becomes even more complex (and perhaps too complex for our purposes) when we consider molecules - combination of atoms bonded together. In molecules, atoms are bonded together as protons in one atom attract the electrons in the clouds of another atom. This electrostatic attraction results in a bond between the two atoms. Electrons are shared by the two atoms as they begin to overlap their electron clouds. If the atoms are of different types (for instance, one atom is Hydrogen and the other atom is Oxygen), then the electrons within the clouds of the two atoms are not equally  shared by the atoms. The clouds become distorted, with the electrons having the greatest probability of being found closest to the more electron-greedy atom. The bond is said to be a polar bond. The distribution of electrons within the cloud is shifted more towards one atom than towards the other atom. This is the case for the two hydrogen-oxygen bonds in the water molecule. Electrons shared by these two atoms are drawn more towards the oxygen atom than towards the hydrogen atom. Subsequently, there is a separation of charge, with oxygen having a partially negative charge and hydrogen having a partially positive charge.
shared by the atoms. The clouds become distorted, with the electrons having the greatest probability of being found closest to the more electron-greedy atom. The bond is said to be a polar bond. The distribution of electrons within the cloud is shifted more towards one atom than towards the other atom. This is the case for the two hydrogen-oxygen bonds in the water molecule. Electrons shared by these two atoms are drawn more towards the oxygen atom than towards the hydrogen atom. Subsequently, there is a separation of charge, with oxygen having a partially negative charge and hydrogen having a partially positive charge.
It is very common to observe this polarization within molecules. In molecules that have long chains of atoms bonded together, there are often several locations along the chain or near the ends of the chain that have polar bonds. This polarization leaves the molecule with areas that have a concentration of positive charges and other areas with a concentration of negative charges. This principle is utilized in the manufacture of certain commercial products that are used to reduce static cling. The centers of positive and negative charge within the product are drawn to excess charge residing on the clothes. There is a neutralization of the static charge buildup on the clothes, thus reducing their tendency to be attracted to each other. (Other products actually use a different principle. During manufacturing, a thin sheet is soaked in a solution containing positively charged ions. The sheet is tossed into the dryer with the clothes. Being saturated with positive charges, the sheet is capable of attracting excess electrons that are scuffed off of clothes during the drying cycle.)
How Does Polarization Explain the Balloon and the Wall Demonstration?
A complete discussion of the world of atoms, molecules and chemical bonds is beyond the scope of The Physics Classroom. Nonetheless, a model of the atom as a distortable cloud of negative electrons surrounding a positive nucleus becomes essential to understanding how an insulating material can be polarized. If a charged object is brought near an insulator, the charges on that object are capable of distorting the electron clouds of the insulator atoms. There is a polarization of the neutral atoms. As shown in the diagrams below, the neutral atoms of the insulator will orient themselves in such a manner as to place the more attractive charge nearest the charged object. Once polarized in this manner, opposites can now attract.

A common demonstration performed in class involved bringing a negatively charged balloon near a wooden door or wooden cabinet. The molecules of wood will reorient themselves in such a way as to place their  positive charges towards the negatively charged balloon. The distortion of their electron clouds will result in an alignment of the wood molecules in a manner that makes the wooden cabinet attracted to the negatively charged balloon. In human terms, one might say that the wood does some quick grooming and then places its most attractive side towards the balloon and its most repulsive side away from the balloon. In the world of static electricity, closeness counts. The negative balloon is closer to the positive portion of the wood molecules and further from the more repulsive negative portion. The balloon and the wall attract with sufficient force to cause the balloon to stick to the wall. From a mechanics standpoint, we would say that the balloon and the wall are pressed together with a large force. The large normal force on the balloon results in a large static friction force. This friction force balances the downward force of gravity and the balloon remains at rest.
positive charges towards the negatively charged balloon. The distortion of their electron clouds will result in an alignment of the wood molecules in a manner that makes the wooden cabinet attracted to the negatively charged balloon. In human terms, one might say that the wood does some quick grooming and then places its most attractive side towards the balloon and its most repulsive side away from the balloon. In the world of static electricity, closeness counts. The negative balloon is closer to the positive portion of the wood molecules and further from the more repulsive negative portion. The balloon and the wall attract with sufficient force to cause the balloon to stick to the wall. From a mechanics standpoint, we would say that the balloon and the wall are pressed together with a large force. The large normal force on the balloon results in a large static friction force. This friction force balances the downward force of gravity and the balloon remains at rest.
Another common physics (and chemistry) demonstration involves using a charged object to deflect a stream of water from its path. Most often, a comb is charged negatively by combing one's hair or a rubber balloon is charged in a similar manner. The negatively charged object is then brought near to a falling stream of water, causing the stream to be attracted to the comb or balloon and alter its direction of fall. The demonstration illustrates the polar nature of water molecules. The hydrogen atoms serve as the positive poles within a water molecule; oxygen serves as the negative pole. Molecules of a liquid are free to rotate and move about; the water molecules realign themselves in order to put their positive poles towards the negatively charged object. Once polarized, the stream and the balloon (or comb) are attracted. As the water molecules within the stream fall past the balloon, this realignment of individual molecules happens quickly and the entire stream is deflected from its original downward direction.

Examples of the attraction between charged objects and neutral objects are numerous and often demonstrated by physics teachers. Paper bits become polarized and are attracted to a charged piece of acetate. Small penguins cut from a sheet of paper are attracted to a charged plastic golf tube and demonstrate their happy feet. A long wooden 2x4 is placed on a pivot and becomes polarized and attracted to a charged golf tube. To the astonishment of students, the force of attraction on the wood is large enough to rotate it about the pivot point.
Polarization is Not Charging
Perhaps the biggest misconception that pertains to polarization is the belief that polarization involves the charging of an object. Polarization is not charging! When an object becomes polarized, there is 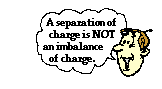 simply a redistribution of the centers of positive and negative charges within the object. Either by the movement of electrons across the surface of the object (as is the case in conductors) or through the distortion of electron clouds (as is the case in insulators), the centers of positive and negative charges become separated from each other. The atoms at one location on the object possess more protons than electrons and the atoms at another location have more electrons than protons. While there are the same number of protons and electrons within the object, these protons and electrons are not distributed in the same proportion across the object's surface. Yet, there are still equal numbers of positive charges (protons) and negative charges (electrons) within the object. While there is a separation of charge, there is NOT an imbalance of charge. When neutral objects become polarized, they are still neutral objects.
simply a redistribution of the centers of positive and negative charges within the object. Either by the movement of electrons across the surface of the object (as is the case in conductors) or through the distortion of electron clouds (as is the case in insulators), the centers of positive and negative charges become separated from each other. The atoms at one location on the object possess more protons than electrons and the atoms at another location have more electrons than protons. While there are the same number of protons and electrons within the object, these protons and electrons are not distributed in the same proportion across the object's surface. Yet, there are still equal numbers of positive charges (protons) and negative charges (electrons) within the object. While there is a separation of charge, there is NOT an imbalance of charge. When neutral objects become polarized, they are still neutral objects.
The process of polarization is often used in many charging methods. In one section of Lesson 2, the charging by induction process will be discussed. This charging process depends upon a charged object to induce polarization within a neutral object. While charging by induction includes polarization as one of its steps, polarization is still NOT a charging process. Details about the induction charging method can be read about in Lesson 2 of this unit.
Triboelectric Charging
How Triboelectric Charging Works
The triboelectic charging process (a.k.a., charging by friction) results in a transfer of electrons between the two objects that are rubbed together. Rubber has a much greater attraction for electrons than animal fur. As a result, the atoms of rubber pull electrons from the atoms of animal fur, leaving both objects with an imbalance of charge. The rubber balloon has an excess of electrons and the animal fur has a shortage of electrons. Having an excess of electrons, the rubber balloon is charged negatively. Similarly, the shortage of electrons on the animal fur leaves it with a positive charge. The two objects have become charged with opposite types of charges as a result of the transfer of electrons from the least electron-loving material to the most electron-loving material.
Triboelectic charging is often demonstrated in Physics class. Two rubber balloons can be suspended from the ceiling and hung at approximately head height. When rubbed upon a teacher's head, the balloons became charged as electrons are transferred from the teacher's fur to the balloons. Since the teacher's fur lost electrons, it became positively charged and the subsequent attraction between the two rubbed objects could be observed. Of course, when the teacher pulls away from the balloons, the balloons experienced a repulsive interaction for each other.

 As mentioned, different materials have different affinities for electrons. By rubbing a variety of materials against each other and testing their resulting interaction with objects of known charge, the tested materials can be ordered according to their affinity for electrons. Such an ordering of substances is known as a triboelectric series. One such ordering for several materials is shown in the table at the right. Materials shown highest on the table tend to have a greater affinity for electrons than those below it. Subsequently, when any two materials in the table are rubbed together, the one that is higher can be expected to pull electrons from the material that is lower. As such, the materials highest on the table will have the greatest tendency to acquire the negative charge. Those below it would become positively charged.
As mentioned, different materials have different affinities for electrons. By rubbing a variety of materials against each other and testing their resulting interaction with objects of known charge, the tested materials can be ordered according to their affinity for electrons. Such an ordering of substances is known as a triboelectric series. One such ordering for several materials is shown in the table at the right. Materials shown highest on the table tend to have a greater affinity for electrons than those below it. Subsequently, when any two materials in the table are rubbed together, the one that is higher can be expected to pull electrons from the material that is lower. As such, the materials highest on the table will have the greatest tendency to acquire the negative charge. Those below it would become positively charged.
The Law of Conservation of Charge
The triboelectic charging process (as well as any charging process) involves a transfer of electrons between two objects. Charge is not created from nothing. The appearance of negative charge upon a rubber balloon is merely the result of its acquisition of electrons. And these electrons must come from somewhere; in this case, from the object it was rubbed against. Electrons are transferred in any charging process. In the case of triboelectic charging, they are transferred between the two objects being rubbed together. Prior to the charging, both objects are electrically neutral. The net charge of the system is 0 units. After the charging process, the more electron-loving object may acquire a charge of -12 units; the other object acquires a charge of +12 units. Overall, the system of two objects has a net charge of 0 units. Whenever a quantity like charge (or momentum or energy or matter) is observed to be the same prior to and after the completion of a given process, we say that the quantity is conserved. Charge is always conserved. When all objects involved are considered prior to and after a given process, we notice that the total amount of charge amidst the objects is the same before the process starts as it is after the process ends. This is referred to as the law of conservation of charge.
Charging By Induction
Charging a Two-Sphere System Using a Negatively Charged Object
 One common demonstration performed in a physics classroom involves the induction charging of two metal spheres. The metal spheres are supported by insulating stands so that any charge acquired by the spheres cannot travel to the ground. The spheres are placed side by side (see diagram i. below) so as to form a two-sphere system. Being made of metal (a conductor), electrons are free to move between the spheres - from sphere A to sphere B and vice versa. If a rubber balloon is charged negatively (perhaps by rubbing it with animal fur) and brought near the spheres, electrons within the two-sphere system will be induced to move away from the balloon. This is simply the principle that like charges repel. Being charged negatively, the electrons are repelled by the negatively charged balloon. And being present in a conductor, they are free to move about the surface of the conductor. Subsequently, there is a mass migration of electrons from sphere A to sphere B. This electron migration causes the two-sphere system to be polarized (see diagram ii. below). Overall, the two-sphere system is electrically neutral. Yet the movement of electrons out of sphere A and into sphere B separates the negative charge from the positive charge. Looking at the spheres individually, it would be accurate to say that sphere A has an overall positive charge and sphere B has an overall negative charge. Once the two-sphere system is polarized, sphere B is physically separated from sphere A using the insulating stand. Having been pulled further from the balloon, the negative charge likely redistributes itself uniformly about sphere B (see diagram iii. below). Meanwhile, the excess positive charge on sphere A remains located near the negatively charged balloon, consistent with the principle that opposite charges attract. As the balloon is pulled away, there is a uniform distribution of charge about the surface of both spheres (see diagram iv. below). This distribution occurs as the remaining electrons in sphere A move across the surface of the sphere until the excess positive charge is uniformly distributed. (This distribution of positive charge on a conductor was discussed in detail earlier in Lesson 1.)
One common demonstration performed in a physics classroom involves the induction charging of two metal spheres. The metal spheres are supported by insulating stands so that any charge acquired by the spheres cannot travel to the ground. The spheres are placed side by side (see diagram i. below) so as to form a two-sphere system. Being made of metal (a conductor), electrons are free to move between the spheres - from sphere A to sphere B and vice versa. If a rubber balloon is charged negatively (perhaps by rubbing it with animal fur) and brought near the spheres, electrons within the two-sphere system will be induced to move away from the balloon. This is simply the principle that like charges repel. Being charged negatively, the electrons are repelled by the negatively charged balloon. And being present in a conductor, they are free to move about the surface of the conductor. Subsequently, there is a mass migration of electrons from sphere A to sphere B. This electron migration causes the two-sphere system to be polarized (see diagram ii. below). Overall, the two-sphere system is electrically neutral. Yet the movement of electrons out of sphere A and into sphere B separates the negative charge from the positive charge. Looking at the spheres individually, it would be accurate to say that sphere A has an overall positive charge and sphere B has an overall negative charge. Once the two-sphere system is polarized, sphere B is physically separated from sphere A using the insulating stand. Having been pulled further from the balloon, the negative charge likely redistributes itself uniformly about sphere B (see diagram iii. below). Meanwhile, the excess positive charge on sphere A remains located near the negatively charged balloon, consistent with the principle that opposite charges attract. As the balloon is pulled away, there is a uniform distribution of charge about the surface of both spheres (see diagram iv. below). This distribution occurs as the remaining electrons in sphere A move across the surface of the sphere until the excess positive charge is uniformly distributed. (This distribution of positive charge on a conductor was discussed in detail earlier in Lesson 1.)

The Law of Conservation of Charge
The law of conservation of charge is easily observed in the induction charging process. Considering the example above, one can look at the two spheres as a system. Prior to the charging process, the overall charge of the system was zero. There were equal numbers of protons and electrons within the two spheres. In diagram ii. above, electrons were induced into moving from sphere A to sphere B. At this point, the individual spheres become charged. The quantity of positive charge on sphere A equals the quantity of negative charge on sphere B. If sphere A has 1000 units of positive charge, then sphere B has 1000 units of negative charge. Determining the overall charge of the system is easy arithmetic; it is simply the sum of the charges on the individual spheres.
Overall Charge of Two Spheres = +1000 units + (-1000 units) = 0 units
The overall charge on the system of two objects is the same after the charging process as it was before the charging process. Charge is neither created nor destroyed during this charging process; it is simply transferred from one object to the other object in the form of electrons.
Charging a Two-Sphere System Using a Positively Charged Object
The above examples show how a negatively charged balloon is used to polarize a two-sphere system and ultimately charge the spheres by induction. But what would happen to sphere A and sphere B if a positively charged object was used to first polarize the two-sphere system? How would the outcome be different and how would the electron movement be altered?
Consider the graphic below in which a positively charged balloon is brought near Sphere A. The presence of the positive charge induces a mass migration of electrons from sphere B towards (and into) sphere A. This movement is induced by the simple principle that opposites attract. Negatively charged electrons throughout the two-sphere system are attracted to the positively charged balloon. This movement of electrons from sphere B to sphere A leaves sphere B with an overall positive charge and sphere A with an overall negative charge. The two-sphere system has been polarized. With the positively charged balloon still held nearby, sphere B is physically separated from sphere A. The excess positive charge is uniformly distributed across the surface of sphere B. The excess negative charge on sphere A remains crowded towards the left side of the sphere, positioning itself close to the balloon. Once the balloon is removed, electrons redistribute themselves about sphere A until the excess negative charge is evenly distributed across the surface. In the end, sphere A becomes charged negatively and sphere B becomes charged positively.

This induction charging process can be used to charge a pair of pop cans. It is a simple enough experiment to be repeated at home. Two pop cans are mounted on Styrofoam cups using scotch tape. The cans are placed side-by-side and a negatively charged rubber balloon (having been rubbed with animal fur) is brought near to one of the cans. The presence of the negative charge near a can induces electron movement from Can A to Can B (see diagram). Once the cans are separated, the cans are charged. The type of charge on the cans can be tested by seeing if they attract the negatively charged balloon or repel the negatively charged balloon. Of course, we would expect that Can A (being positively charged) would attract the negatively charged balloon and Can B (being negatively charged) should repel the negatively charged balloon. During the process of induction charging, the role of the balloon is to simply induce a movement of electrons from one can to the other can. It is used to polarize the two-can system. The balloon never does supply electrons to can A (unless your hear a spark, indicating a lightning discharge from the balloon to the can).

The Importance of a Ground in Induction Charging
In the charging by induction cases discussed above, the ultimate charge on the object is never the result of electron movement from the charged object to the originally neutral objects. The balloon never transfers electrons to or receive electrons from the spheres; nor does the glass rod transfer electrons to or receive electrons from the spheres. The neutral object nearest the charged object (sphere A in these discussions) acquires its charge from the object to which it is touched. In the above cases, the second sphere is used to supply the electrons to sphere A or to receive electrons from sphere A. The role of sphere B in the above examples is to serve as a supplier or receiver of electrons in response to the object that is brought near sphere A. In this sense, sphere B acts like a ground.
To further illustrate the importance of a ground, consider the induction charging of a single conducting sphere. Suppose that a negatively charged rubber balloon is brought near a single sphere as shown below (Diagram ii). The presence of the negative charge will induce electron movement in the sphere. Since like charges repel, negative electrons within the metal sphere will be repelled by the negatively charged balloon. There will be a mass migration of electrons from the left side of the sphere to the right side of the sphere causing charge within the sphere to become polarized (Diagram ii). Once charge within the sphere has become polarized, the sphere is touched. The touching of the sphere allows electrons to exit the sphere and move through the hand to "the ground" (Diagram iii). It is at this point that the sphere acquires a charge. With electrons having left the sphere, the sphere acquires a positive charge (Diagram iv). Once the balloon is moved away from the sphere, the excess positive charge redistributes itself (by the movement of remaining electrons) such that the positive charge is uniformly distributed about the sphere's surface.

There are several things to note about this example of induction charging. First, observe that the third step of the process involves the touching of the sphere by a person. The person serves the role of the ground. If compared to the induction charging of a two-sphere system, the person has simply replaced the second sphere (Sphere B). Electrons within the sphere are repelled by the negative balloon and make an effort to distance themselves from it in order to minimize the repulsive affects. (This distance factor will be discussed in great detail in Lesson 3). While these electrons crowd to the right side of the sphere to distance themselves from the negatively charged balloon, they encounter another problem. In human terms, it could be said that the excess electrons on the right side of the sphere not only find the balloon to be repulsive, they also find each other to be repulsive. They simply need more space to distance themselves from the balloon as well as from each other. Quite regrettably for these electrons, they have run out of real estate; they cannot go further than the boundary of the sphere. Too many electrons in the same neighborhood is not a good thing. And when the hand comes nearby, these negative electrons see opportunity to find more real estate - a vast body of a human being into which they can roam and subsequently distance themselves even further from each other. It is in this sense, that the hand and the body to which it is attached (assuming of course that the hand is attached to a body) serve as a ground. A ground is simply a large object that serves as an almost infinite source of electrons or sink for electrons. A ground contains such vast space that it is the ideal object to either receive electrons or supply electrons to whatever object needs to get rid of them or receive them.
The second thing to note about the induction charging process shown above is that the sphere acquires a charge opposite the balloon. This will always be the observed case. If a negatively charged object is used to charge a neutral object by induction, then the neutral object will acquire a positive charge. And if a positively charged object is used to charge a neutral object by induction, then the neutral object will acquire a negative charge. If you understand the induction charging process, you can see why this would always be the case. The charged object that is brought near will always repel like charges and attract opposite charges. Either way, the object being charged acquires a charge that is opposite the charge of the object used to induce the charge. To further illustrate this, the diagram below shows how a positively charged balloon will charge a sphere negatively by induction.

The Electrophorus
A commonly used lab activity that demonstrates the induction charging method is the Electrophorus Lab. In this lab, a flat plate of foam is rubbed with animal fur in order to impart a negative charge to the foam. Electrons are transferred from the animal fur to the more electron-loving foam (Diagram i.). An aluminum pie plate is taped to a Styrofoam cup; the aluminum is a conductor and the Styrofoam serves as an insulating handle. As the aluminum plate is brought near, electrons within the aluminum are repelled by the negatively charged foam plate. There is a mass migration of electrons to the rim of the aluminum pie plate. At this point, the aluminum pie plate is polarized, with the negative charge located along the upper rim farthest from the foam plate (Diagram ii.). The rim of the plate is then touched, providing a pathway from the aluminum plate to the ground. Electrons along the rim are not only repelled by the negative foam plate, they are also repelled by each other. So once touched, there is a mass migration of electrons from the rim to the person touching the rim (Diagram iii.). Being of much greater size than the aluminum pie plate, the person provides more space for the mutually repulsive electrons. The moment that electrons depart from the aluminum plate, the aluminum can be considered a charged object. Having lost electrons, the aluminum possesses more protons than electrons and is therefore positively charged. Once the foam plate is removed, the excess positive charge becomes distributed about the surface of the aluminum plate in order to minimize the overall repulsive forces between them (Diagram iv.).

The Electrophorus Lab further illustrates that when charging a neutral object by induction, the charge imparted to the object is opposite that of the object used to induce the charge. In this case, the foam plate was negatively charged and the aluminum plate became positively charged. The lab also illustrates that there is never a transfer of electrons between the foam plate and the aluminum plate. The aluminum plate becomes charged by a transfer of electrons to the ground. Finally, one might note that the role of the charged object in induction charging is to simply polarize the object being charged. This polarization occurs as the negative foam plate repels electrons from the near side, inducing them to move to the opposite side of the aluminum plate. The presence of the positive charge on the bottom of the aluminum plate is the result of the departure of electrons from that location. Protons did not move downwards through the aluminum. The protons were always there from the beginning; it's just that they have lost their electron partners. Protons are fixed in place and incapable of moving in any electrostatic experiment.
The Electroscope
Another common lab experience that illustrates the induction charging method is the Electroscope Lab. In the Electroscope Lab, a positively charged object such as an aluminum pie plate is used to charge an electroscope by induction. An electroscope is a device that is capable of detecting the presence of a charged object. It is often used in electrostatic experiments and demonstrations in order to test for charge and to deduce the type of charge present on an object. There are all kinds of varieties and brands of electroscope from the gold leaf electroscope to the needle electroscope.
While there are different types of electroscopes, the basic operation of each is the same. The electroscope typically consists of a conducting plate or knob, a conducting base and either a pair of conducting leaves or a conducting needle. Since the operating parts of an electroscope are all conducting, electrons are capable of moving from the plate or knob on the top of the electroscope to the needle or leaves in the bottom of the electroscope. Objects are typically touched to or held nearby the plate or knob, thus inducing the movement of electrons into the needle or the leaves (or from the needle/leaves to the plate/knob). The gold leaves or needle of the electroscope are the only mobile parts. Once an excess of electrons (or a deficiency of electrons) is present in the needle or the gold leaves, there will be a repulsive affect between like charges causing the leaves to repel each other or the needle to be repelled by the base that it rests upon. Whenever this movement of the leaves/needle is observed, one can deduce that an excess of charge - either positive or negative - is present there. It is important to note that the movement of the leaves and needle never directly indicate the type of charge on the electroscope; it only indicates that the electroscope is detecting a charge.

Suppose a needle electroscope is used to demonstrate induction charging. An aluminum pie plate is first charged positively by the process of induction (see discussion above). The aluminum plate is then held above the plate of the electroscope. Since the aluminum pie plate is not touched to the electroscope, the charge on the aluminum plate is NOT conducted to the electroscope. Nonetheless, the aluminum pie plate does have an affect upon the electrons in the electroscope. The pie plate induces electrons within the electroscope to move. Since opposites attract, a countless number of negatively charged electrons are drawn upwards towards the top of the electroscope. Having lost numerous electrons, the bottom of the electroscope has a temporarily induced positive charge. Having gained electrons, the top of the electroscope has a temporarily induced negative charge (Diagram ii. below). At this point the electroscope is polarized; however, the overall charge of the electroscope is neutral. The charging step then occurs as the bottom of the electroscope is touched to the ground. Upon touching the bottom of the electroscope, electrons enter the electroscope from the ground. One explanation of their entry is that they are drawn into the bottom of the electroscope by the presence of the positive charge at the bottom of the electroscope. Since opposites attract, electrons are drawn towards the bottom of the electroscope (Diagram iii.). As electrons enter, the needle of the electroscope is observed to return to the neutral position. This needle movement is the result of negative electrons neutralizing the previously positively charged needle at the bottom of the electroscope. At this point, the electroscope has an overall negative charge. The needle does not indicate this charge because the excess of electrons is still concentrated in the top plate of the electroscope; they are attracted to the positively charged aluminum pie plate that is held above the electroscope (Diagram iv.). Once the aluminum pie plate is pulled away, the excess of electrons in the electroscope redistribute themselves about the conducting parts of the electroscope. As they do, numerous excess electrons enter the needle and the base upon which the needle rests. The presence of excess negative charged in the needle and the base causes the needle to deflect, indicating that the electroscope has been charged (Diagram v.).

The above discussion provides one more illustration of the fundamental principles regarding induction charging. These fundamental principles have been illustrated in each example of induction charging discussed on this page. The principles are:
The charged object is never touched to the object being charged by induction.
The charged object does not transfer electrons to or receive electrons from the object being charged.
The charged object serves to polarize the object being charged.
The object being charged is touched by a ground; electrons are transferred between the ground and the object being charged (either into the object or out of it).
The object being charged ultimately receives a charge that is opposite that of the charged object that is used to polarize it.
Charging by Conduction
Charging by conduction involves the contact of a charged object to a neutral object. Suppose that a positively charged aluminum plate is touched to a neutral metal sphere. The neutral metal sphere becomes charged as the result of being contacted by the charged aluminum plate. Or suppose that a negatively charged metal sphere is touched to the top plate of a neutral needle electroscope. The neutral electroscope becomes charged as the result of being contacted by the metal sphere. And finally, suppose that an uncharged physics student stands on an insulating platform and touches a negatively charged Van de Graaff generator. The neutral physics student becomes charged as the result of contact with the Van de Graaff generator. Each of these examples involves contact between a charged object and a neutral object. In contrast to induction, where the charged object is brought near but never contacted to the object being charged, conduction charging involves making the physical connection of the charged object to the neutral object. Because charging by conduction involves contact, it is often called charging by contact.
Charging by Conduction Using a Negatively Charged Object
To explain the process of charging by contact, we will first consider the case of using a negatively charged metal sphere to charge a neutral needle electroscope. Understanding the process demands that you understand that like charges repel and have an intense desire to reduce their repulsions by spreading about as far as possible. A negatively charged metal sphere has an excess of electrons; those electrons find each other repulsive and distance themselves from each other as far as possible. The perimeter the sphere is the extreme to which they can go. If there was ever a conducting pathway to a more spacious piece of real estate, one could be sure that the electrons would be on that pathway to the greener grass beyond. In human terms, electrons living in the same home despise each other and are always seeking a home of their own or at least a home with more rooms.
Given this understanding of electron-electron repulsions, it is not difficult to predict what excess electrons on the metal sphere would be inclined to do if the sphere were touched to the neutral electroscope. Once the contact of the sphere to the electroscope is made, a countless number of excess electrons from the sphere move onto the electroscope and spread about the sphere-electroscope system. In general, the object that offers the most space in which to "hang out" will be the object that houses the greatest number of excess electrons. When the process of charging by conduction is complete, the electroscope acquires an excess negative charge due to the movement of electrons onto it from the metal sphere. The metal sphere is still charged negatively, only it has less excess negative charge than it had prior to the conduction charging process.

Charging by Conduction Using a Positively Charged Object
The previous example of charging by conduction involved touching a negatively charged object to a neutral object. Upon contact, electrons moved from the negatively charged object onto the neutral object. When finished, both objects were negatively charged. But what happens if a positively charged object is touched to a neutral object? To investigate this question, we consider the case of a positively charged aluminum plate being used to charge a neutral metal sphere by the process of conduction.
The diagram below depicts the use of a positively charged aluminum plate being touched to a neutral metal sphere. A positively charged aluminum plate has an excess of protons. When looked at from an electron perspective, a positively charged aluminum plate has a shortage of electrons. In human terms, we could say that each excess proton is rather discontented. It is not satisfied until it has found a negatively charged electron with which to co-habitate. However, since a proton is tightly bound in the nucleus of an atom, it is incapable of leaving an atom in search of that longed-for electron. It can however attract a mobile electron towards itself. And if a conducting pathway is made between a collection of electrons and an excess proton, one can be certain that there is likely an electron that would be willing to take the pathway. So when the positively charged aluminum plate is touched to the neutral metal sphere, countless electrons on the metal sphere migrate towards the aluminum plate. There is a mass migration of electrons until the positive charge on the aluminum plate-metal sphere system becomes redistributed. Having lost electrons to the positively charged aluminum plate, there is a shortage of electrons on the sphere and an overall positive charge. The aluminum plate is still charged positively; only it now has less excess positive charge than it had before the charging process began.

The above explanation might raise a rather difficult question: Why would an electron on the previously neutral metal sphere desire to move off the metal sphere in the first place? The metal sphere is neutral; every electron on it must be satisfied since there is a corresponding proton present. What would possibly induce an electron to go through the effort of migrating to a different territory in order to have what it already has?
The best means of answering this question requires an understanding of the concept of electric potential. But since that concept does not arise until the next unit of The Physics Classroom, a different approach to an answer will be taken. It ends up that electrons and protons are not as independent and individualized as we might think. From a human perspective, electrons and protons can't be thought of as independent citizens in a free enterprise system of government. Electrons and protons don't actually do what is best for themselves, but must be more social-minded. They must act like citizens of a state where the rule of law is to behave in a manner such that the overall repulsive affects within the society at large are reduced and the overall attractive affects are maximized. Electrons and protons will be motivated not by what is good for them, but rather by what is good for the country. And in this sense, a country's boundary extends to the perimeter of the conductor material that an excess electron is within. And in this case, an electron in the metal sphere is part of a country that extends beyond the sphere itself and includes the entire aluminum plate. So by moving from the metal sphere to the aluminum plate, an electron is able to reduce the total amount of repulsive affects within that country. It serves to spread the excess positive charge over a greater surface area, thus reducing the total amount of repulsive forces between excess protons.
Law of Conservation of Charge
In each of the other methods of charging discussed in Lesson 2 - charging by friction and charging by induction - the law of conservation of charge was illustrated. The law of conservation of charge states that charge is always conserved. When all objects involved are considered prior to and after a given process, we notice that the total amount of charge among the objects is the same before the process starts as it is after the process ends. The same conservation law is observed during the charging by conduction process. If a negatively charged metal sphere is used to charge a neutral electroscope, the overall charge before the process begins is the same as the overall charge when the process ends. So if before the charging process begins, the metal sphere has 1000 units of negative charge and the electroscope is neutral, the overall charge of the two objects in the system is -1000 units. Perhaps during the charging process, 600 units of negative charge moved from the metal sphere to the electroscope. When the process is complete, the electroscope would have 600 units of negative charge and the metal sphere would have 400 units of negative charge (the original 1000 units minus the 600 units it transferred to the electroscope). The overall charge of the two objects in the system is still -1000 units. The overall charge before the process began is the same as the overall charge when the process is completed. Charge is neither created nor destroyed; it is simply transferred from one object to another object in the form of electrons.
Conduction Charging Requires a Conductor
In all the above examples, the charging by conduction process involved the touching of two conductors. Does contact charging have to occur through the contact of two conductors? Can an insulator conduct a charge to another object upon touching? And can an insulator be charged by conduction? A complete discussion of these questions can get messy and quite often leads to a splitting of hairs over the definition of conduction and the distinction between conductors and  insulators. The belief is taken here that only a conductor can conduct charge to another conductor. The process of noticeably charging an object by contact involves the two contacting objects momentarily sharing the net excess charge. The excess charge is simply given a larger area over which to spread in order to reduce the total amount of repulsive forces between them. This process demands that the objects be conductors in order for electrons to move about and redistribute themselves. An insulator hinders such a movement of electrons between touching objects and about the surfaces of the objects. This is observed if an aluminum pie plate is placed upon a charged foam plate. When the neutral aluminum plate is placed upon the charged foam plate, the foam plate does not conduct its charge to the aluminum. Despite the fact that the two surfaces were in contact, charging by contact or conduction did not occur. (Or at least whatever charge transfer might have occurred was not noticeable by the customary means of using an electroscope, using a charge testing bulb or testing for its repulsion with a like-charged object.)
insulators. The belief is taken here that only a conductor can conduct charge to another conductor. The process of noticeably charging an object by contact involves the two contacting objects momentarily sharing the net excess charge. The excess charge is simply given a larger area over which to spread in order to reduce the total amount of repulsive forces between them. This process demands that the objects be conductors in order for electrons to move about and redistribute themselves. An insulator hinders such a movement of electrons between touching objects and about the surfaces of the objects. This is observed if an aluminum pie plate is placed upon a charged foam plate. When the neutral aluminum plate is placed upon the charged foam plate, the foam plate does not conduct its charge to the aluminum. Despite the fact that the two surfaces were in contact, charging by contact or conduction did not occur. (Or at least whatever charge transfer might have occurred was not noticeable by the customary means of using an electroscope, using a charge testing bulb or testing for its repulsion with a like-charged object.)
Many might quickly suggest that they have used a charged insulator to charge a neutral electroscope (or some other object) by contact. In fact, a negatively charged plastic golf tube can used to charge an electroscope. The plastic tube is touched to the top plate of the electroscope. On most occasions, the plastic tube is even rubbed or rolled across the plate of the electroscope? Wouldn't this be regarded as charging by conduction? No. Not really. In this case, it is more than  likely that the charging occurred by some process other than conduction. There was not a sharing of charge between the plastic tube and the metal parts of the electroscope. Of course, once some excess charge is acquired by the electroscope, that excess charge distributes itself about the surface of the electroscope. Yet the charge is not uniformly shared between the two objects. The protons and electrons within both the plastic golf tube and the electroscope are not acting together to share excess charge and reduce the total amount of repulsive forces.
likely that the charging occurred by some process other than conduction. There was not a sharing of charge between the plastic tube and the metal parts of the electroscope. Of course, once some excess charge is acquired by the electroscope, that excess charge distributes itself about the surface of the electroscope. Yet the charge is not uniformly shared between the two objects. The protons and electrons within both the plastic golf tube and the electroscope are not acting together to share excess charge and reduce the total amount of repulsive forces.
The charging of an electroscope by contact with a negatively charged golf tube (or any charged insulating object) would best be described as charging by lightning. Rather than being a process in which the two objects act together to share the excess charge, the process could best be described as the successful effort of electrons to burst through the space (air) between objects. The presence of a negatively charged plastic tube is capable of ionizing the air surrounding the tube and allowing excess electrons on the plastic tube to be conducted through the air to the electroscope. This transfer of charge can happen with or without touching. In fact, on a dry winter day the process of charging the metal electroscope with the charged insulator often occurs while the insulator is some distance away. The dry air is more easily ionized and a greater quantity of electrons is capable of bursting through the space between the two objects. On such occasions, a crackling sound is often heard and a flash of light is seen if the room is darkened. This phenomenon, occurring from several centimeters away, certainly does not fit the description of contact charging.
A charged insulating object is certainly capable of transferring its charge to another object. The result of the charge transfer will be the same as the result of charging by conduction. Both objects will have the same type of charge and the flow of electrons is in the same direction. However, the process and the underlying explanations are considerably different. In the case of charging an object with a charged insulator, the contact is not essential. Contacting the object simply reduces the spatial separation between touching atoms and allows charge to arc and spark its way between objects. Rubbing or rolling the insulating object across the conductor's surface facilitates the charging process by bringing a greater number of atoms on the insulator in close proximity to the conductor that is receiving the charge. The two materials do not make any effort to share charge nor to act as a single object (with a uniform electric potential) in an effort to reduce repulsive affects.
Is this distinction between charging by conduction and charging by lightning a splitting of hairs? Perhaps. For certain, each process involves a transfer of charge from one object to another object, yielding the same result - two like-charged object. Yet, distinguishing between the two forms of charging is more consistent with the customary view that insulators are not conductors of charge. It also serves to explain why some insulators clearly do not always transfer their charge upon contact. This phenomenon of charging by lightning will be revisited in Lesson 4 during a discussion of electric fields and lightning discharges.
Grounding
The previous three sections of Lesson 2 discussed the three common methods of charging - charging by friction, charging by induction, and charging by conduction. A discussion of charging would not be complete without a discussion of uncharging. Objects with an excess of charge - either positive or negative - can have this charge removed by a process known as grounding. Grounding is the process of removing the excess charge on an object by means of the transfer of electrons between it and another object of substantial size. When a charged object is grounded, the excess charge is balanced by the transfer of electrons between the charged object and a ground. A ground is simply an object that serves as a seemingly infinite reservoir of electrons; the ground is capable of transferring electrons to or receiving electrons from a charged object in order to neutralize that object. In this last section of Lesson 2, the process of grounding will be discussed.
Grounding a Negatively Charged Object
To begin our discussion of grounding, we will consider the grounding of a negatively charged electroscope. Any negatively charged object has an excess of electrons. If it is to have its charge  removed, then it will have to lose its excess electrons. Once the excess electrons are removed from the object, there will be equal numbers of protons and electrons within the object and it will have a balance of charge. To remove the excess of electrons from a negatively charged electroscope, the electroscope will have to be connected by a conducting pathway to another object that is capable of receiving those electrons. The other object is the ground. In typical electrostatic experiments and demonstrations, this is simply done by touching the electroscope with one's hand. Upon contact, the excess electrons leave the electroscope and enter the person who touches it. These excess electrons subsequently spread about the surface of the person.
removed, then it will have to lose its excess electrons. Once the excess electrons are removed from the object, there will be equal numbers of protons and electrons within the object and it will have a balance of charge. To remove the excess of electrons from a negatively charged electroscope, the electroscope will have to be connected by a conducting pathway to another object that is capable of receiving those electrons. The other object is the ground. In typical electrostatic experiments and demonstrations, this is simply done by touching the electroscope with one's hand. Upon contact, the excess electrons leave the electroscope and enter the person who touches it. These excess electrons subsequently spread about the surface of the person.
This process of grounding works because excess electrons find each other repulsive. As is always the case, repulsive affects between like-charged electrons forces them to look for a means of spatially separating themselves from each other. This spatial separation is achieved by moving to a larger object that allows a greater surface area over which to spread. Because of the relative size of a person compared to a typical electroscope, the excess electrons (nearly all of them) are capable of reducing the repulsive forces by moving into the person (i.e., the ground). Like contact charging discussed earlier, grounding is simply another example of charge sharing between two objects. The extent to which an object is willing to share excess charge is proportional to its size. So an effective ground is simply an object with significant enough size to share the overwhelming majority of excess charge.
Grounding a Positively Charged Object
The previous discussion describes the grounding of a negatively charged electroscope. Electrons were transferred from the  electroscope to the ground. But what if the electroscope is positively charged? How does electron transfer allow an object with an excess of protons to become neutralized? To explore these questions, we will consider the grounding of a positively charged electroscope. A positively charged electroscope must gain electrons in order to acquire an equal number of protons and electrons. By gaining electrons from the ground, the electroscope will have a balance of charge and therefore be neutral. Thus, the grounding of a positively charged electroscope involves the transfer of electrons from the ground into the electroscope. This process works because excess positive charge on the electroscope attracts electrons from the ground (in this case, a person). While this may disrupt any balance of charge present on the person, the significantly larger size of the person allows for the excess charge to distance itself further from each other. As in the case of grounding a negatively charged electroscope, the grounding of a positively charged electroscope involves charge sharing. The excess positive charge is shared between the electroscope and the ground. And once again, the extent to which an object is willing to share excess charge is proportional to its size. The person is an effective ground because it has enough size to share the overwhelming majority of excess positive charge.
electroscope to the ground. But what if the electroscope is positively charged? How does electron transfer allow an object with an excess of protons to become neutralized? To explore these questions, we will consider the grounding of a positively charged electroscope. A positively charged electroscope must gain electrons in order to acquire an equal number of protons and electrons. By gaining electrons from the ground, the electroscope will have a balance of charge and therefore be neutral. Thus, the grounding of a positively charged electroscope involves the transfer of electrons from the ground into the electroscope. This process works because excess positive charge on the electroscope attracts electrons from the ground (in this case, a person). While this may disrupt any balance of charge present on the person, the significantly larger size of the person allows for the excess charge to distance itself further from each other. As in the case of grounding a negatively charged electroscope, the grounding of a positively charged electroscope involves charge sharing. The excess positive charge is shared between the electroscope and the ground. And once again, the extent to which an object is willing to share excess charge is proportional to its size. The person is an effective ground because it has enough size to share the overwhelming majority of excess positive charge.
The Need for a Conducting Pathway
Any object can be grounded provided that the charged atoms of that object have a conducting pathway between the atoms and the ground. A common lab activity involves taping two straws to a charged aluminum plate. One straw is covered with aluminum foil and the other straw is bare plastic. When the aluminum-covered straw is touched, the aluminum plate loses its charge. It is grounded by means of the movement of electrons from the ground to the aluminum plate. When the plastic straw is touched, grounding does not occur. The plastic serves as an insulator and prevents the flow of electrons from the ground to the aluminum plate. Grounding requires a conducting pathway between the ground and the object to be grounded. Electrons will travel along that pathway.
 Knowt
Knowt

