
Chapter 6: Separation Methods
6.1: Liquid Phase Extraction
Liquid Phase Extraction - It involves the separation of two or more substances in an analyte through a process where two solvents are employed.
The two solvents are immiscible.
Partitioning – The competition between two solvents for an analyte. This involves analytes being distributed between the two solvents according to certain chemical properties, mainly polarity and pH.
Polarity
Polarity - it is the tendency of the compound to behave like a miniature magnet, with a positive side and a negative side.
It is caused by an excess of electron density on one side of a molecule and therefore a deficiency on the other side.
The side with the excess electron density has a net negative charge and the other side has a net positive charge because it is deficient in electrons.
pH
Acidic Substance - releases hydrogen ions H+ (they become hydrated in the presence of water, so they are in the form of H2O. H+ or H3O+ called hydronium ions) when dissolved in water.
Its pH is between 0 and 7.
Alkaline/Basic Substance - releases hydroxide (OH−) ions when dissolved in water.
Its pH is between 7 and 14.
Neutral substance - releases neither H3O+ nor OH− ions when dissolved.
Its pH is 7.
Liquid phase extractions are commonly used to separate mixtures of solids. They are ideal for separating substances where one is much more soluble than the rest or if they are of different polarity and pH.
6.2: Solid Phase Extraction
Solid phase extraction does not involve a partitioning mechanism.
It relies on adsorption — a process whereby a solid, liquid, or gaseous analyte is attracted to the surface of a solid adsorbing material. The analyte molecules adhere to its surface.
Solid Phase Microextraction – This technique is used for very small samples.
A small wire is coated with an adsorbent such as charcoal and attached to a holder that can extend or withdraw the wire. The wire is extended into a vapor or liquid where adsorption takes place. Then the wire can be introduced into a gas chromatograph where the adsorbed materials are eluted and analyzed.
6.3: Chromatography
A Russian botanist Michael Tswett revolutionized analytical chemistry by developing a separation method that has evolved into a whole family of analytical techniques applicable to a huge variety of mixtures of solids, liquids, and vapors, over a large temperature range, whether they are soluble in solvents or not. This family is collectively known as chromatography.
Two Phases of Chromatography:
Stationary Phase – a finely divided solid material or a viscous liquid that is contained within a long column.
Mobile Phase – a liquid or a gas under pressure. It moves through the stationary phase carrying the analyte mixture with it.
6.4: Gas Chromatography
Gas Chromatography - It is the most versatile chromatography method.
Modern GC is called capillary GC because the stationary phase is contained within a very narrow, hollow tube that is made of glass or a synthetic polymer and is often coated with a plastic to add strength.
The mobile phase in GC is an inert gas.
The most commonly used gas is helium.
Nitrogen is also used, but gives inferior results.
The best gas is hydrogen, but it is more expensive than helium and is quite flammable.
Forensically important substances that are not stable at high temperatures include many explosives. The presence of the inert gas as the mobile phase purges the entire system of oxygen, so there is no chance for analytes to burn when they are heated inside the instrument.
Parts of the Gas Chromatograph
Gas Chromatograph – an instrument that is used to separate components of an analyte mixture using the principles of GC.
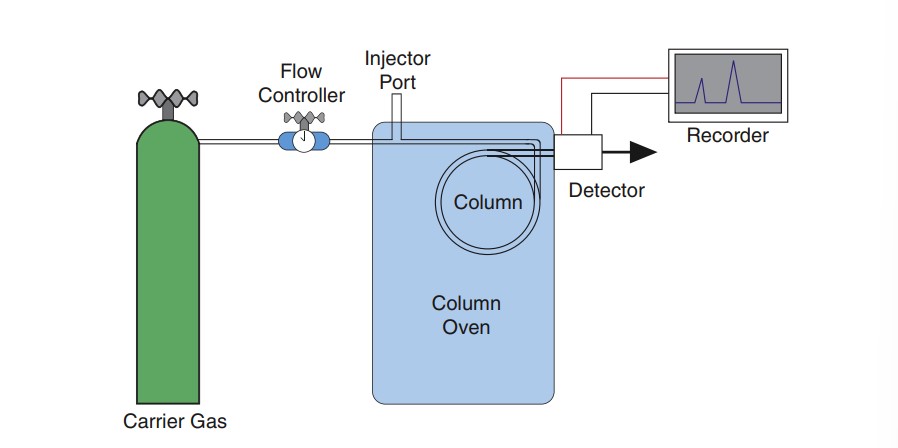
GC Detectors
Flame Ionization Detector – produces a small flame from the reaction of hydrogen taken from a tank and oxygen from the air.
Mass Spectrometer Detector – can detect and identify each analyte component as it elutes from the column.
Nitrogen-Phosphorous Detector – it can detect only substances that contain nitrogen or phosphorous. This type is widely used in biological, toxicological, and environmental applications and is very sensitive.
Thermal conductivity Detector – relies on the change in the ability of the mobile phase gas to conduct heat as it is mixed with an analyte. It is simple to engineer and use and is very versatile.
Electron Capture Detector – an extremely sensitive detector that is used on substances that have a halide such as a chloride or bromide, or oxygen in the molecule.
Pyrolysis Gas Chromatography – a modification of GC making it possible for a gas chromatograph to analyze polymers.
Pyrolysis – to heat in the absence of air.
If a polymer such as fiber is heated to very high temperatures, up to 1000°C, in the absence of oxygen, it will not burn but instead will decompose into stable fragments, called pyrolyzates.
Fourier transform infrared spectrophotometer – the GC separates a mixture into individual components and then each one can be introduced into a special sample compartment in an FTIR.
6.5: High-Performance Liquid Chromatography
Stationary phases have become much more efficient in separating components of an analyte, and they are much more sensitive.
The process can be sped up by having the mobile phase pushed through the stationary phase using pumps.
This makes the experiment go much faster while keeping the high resolving power of the technique.
Gradient Chromatography – the composition of the mobile phase can be altered during the run.
Isocratic Chromatography– the mobile phase stays constant during an HPLC run.
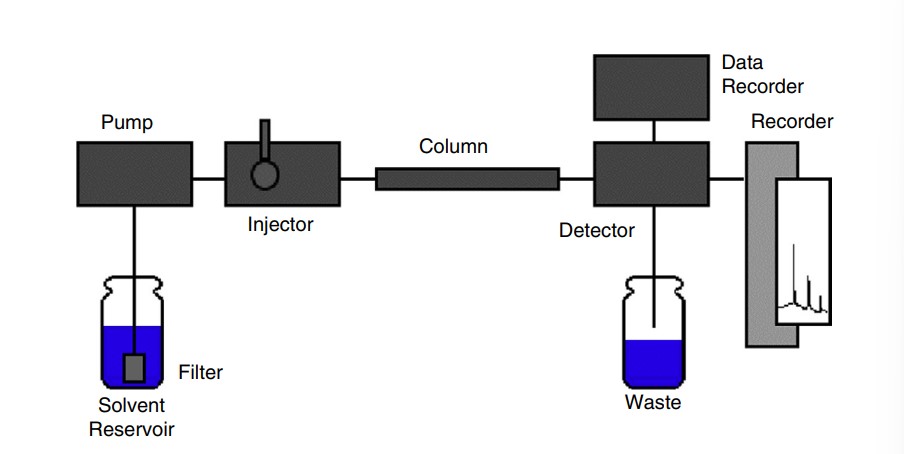
HPLC Detectors
Diode array detector – measures the ultraviolet and visible spectrum of the solution as it flows through.
Fluorescence Detector – detect only those substances that exhibit fluorescence, such as the illicit drug LSD. This limits its utility, but it is extremely sensitive. Ultraviolet or visible light is used as the source.
Conductivity detector – detects only conductors.
RI Detector – measures a mobile phase with an analyte dissolved in it will refract light differently than the mobile phase alone.
Mass Spectrometry
When the mobile phase and analytes reach the mass spectrometer, the mobile phase is stripped away and the mass spectrum of each component of the analyte is measured. As in GC, this permits separations and identifications to take place in one step.
Application of HPLC
Drugs
Drugs are more often identified by GC because of the ease with which a mass spectrometer can be used.
Although HPLC/MS instruments are now commercially available, they are not widely used in forensic science laboratories.
Soils
Organic extractions can be done on soils and the various substances can be separated. The result is a profile of the soil.
The substances in the mixture are usually not identified, but the profile is a useful way of determining if soil found at a crime scene could have come from a particular location.
Explosives
It may not be safe to run explosive extracts by GC because of the high heat, but HPLC is an ideal method for the separation of explosive residues.
Inks and dyes:
Determination of the visible and UV spectra of inks is useful in comparing a writing instrument to writing on a document.
It can also be used to follow the aging of the ink as it dries and degrades.
Fiber dyes can be extracted from fibers and separated by HPLC also.
6.6: Thin Layer Chromatography
Paper chromatography is one of the oldest methods of separation and is still used in some applications. The paper acts as a stationary phase and the ink solvent is the mobile phase.
Thin Layer Chromatography — a technique used to isolate non-volatile mixtures.
The experiment is conducted on a sheet of aluminum foil, plastic, or glass which is coated with a thin layer of adsorbent material. The material usually used is aluminum oxide, cellulose, or silica gel.
6.7: Electrophoresis
Two major types of electrophoresis:
Gel electrophoresis – is similar in some respects to TLC; a technique used to separate DNA fragments according to their size.
Capillary electrophoresis – is similar to HPLC; the primary methodology used for separating and detecting short tandem repeat (STR) alleles in forensic DNA laboratories worldwide.
Application of Electrophoresis
DNA Typing
The only method available for separating DNA fragments suitable for forensic DNA typing. Either gel or capillary electrophoresis can be used.
Drugs
Capillary electrophoresis is the most sensitive of the methods.
Explosive Residues
Capillary electrophoresis has been employed because it is more sensitive and has a higher resolution than HPLC. Most organic explosives can be separated by this method.
Gunshot residues
Capillary electrophoresis is very sensitive. It must be optimized for the detection of inorganic substances in this application
Questioned Documents
Capillary electrophoresis has just begun to be used for the separation of ink components used in pens.
Chapter 6: Separation Methods
6.1: Liquid Phase Extraction
Liquid Phase Extraction - It involves the separation of two or more substances in an analyte through a process where two solvents are employed.
The two solvents are immiscible.
Partitioning – The competition between two solvents for an analyte. This involves analytes being distributed between the two solvents according to certain chemical properties, mainly polarity and pH.
Polarity
Polarity - it is the tendency of the compound to behave like a miniature magnet, with a positive side and a negative side.
It is caused by an excess of electron density on one side of a molecule and therefore a deficiency on the other side.
The side with the excess electron density has a net negative charge and the other side has a net positive charge because it is deficient in electrons.
pH
Acidic Substance - releases hydrogen ions H+ (they become hydrated in the presence of water, so they are in the form of H2O. H+ or H3O+ called hydronium ions) when dissolved in water.
Its pH is between 0 and 7.
Alkaline/Basic Substance - releases hydroxide (OH−) ions when dissolved in water.
Its pH is between 7 and 14.
Neutral substance - releases neither H3O+ nor OH− ions when dissolved.
Its pH is 7.
Liquid phase extractions are commonly used to separate mixtures of solids. They are ideal for separating substances where one is much more soluble than the rest or if they are of different polarity and pH.
6.2: Solid Phase Extraction
Solid phase extraction does not involve a partitioning mechanism.
It relies on adsorption — a process whereby a solid, liquid, or gaseous analyte is attracted to the surface of a solid adsorbing material. The analyte molecules adhere to its surface.
Solid Phase Microextraction – This technique is used for very small samples.
A small wire is coated with an adsorbent such as charcoal and attached to a holder that can extend or withdraw the wire. The wire is extended into a vapor or liquid where adsorption takes place. Then the wire can be introduced into a gas chromatograph where the adsorbed materials are eluted and analyzed.
6.3: Chromatography
A Russian botanist Michael Tswett revolutionized analytical chemistry by developing a separation method that has evolved into a whole family of analytical techniques applicable to a huge variety of mixtures of solids, liquids, and vapors, over a large temperature range, whether they are soluble in solvents or not. This family is collectively known as chromatography.
Two Phases of Chromatography:
Stationary Phase – a finely divided solid material or a viscous liquid that is contained within a long column.
Mobile Phase – a liquid or a gas under pressure. It moves through the stationary phase carrying the analyte mixture with it.
6.4: Gas Chromatography
Gas Chromatography - It is the most versatile chromatography method.
Modern GC is called capillary GC because the stationary phase is contained within a very narrow, hollow tube that is made of glass or a synthetic polymer and is often coated with a plastic to add strength.
The mobile phase in GC is an inert gas.
The most commonly used gas is helium.
Nitrogen is also used, but gives inferior results.
The best gas is hydrogen, but it is more expensive than helium and is quite flammable.
Forensically important substances that are not stable at high temperatures include many explosives. The presence of the inert gas as the mobile phase purges the entire system of oxygen, so there is no chance for analytes to burn when they are heated inside the instrument.
Parts of the Gas Chromatograph
Gas Chromatograph – an instrument that is used to separate components of an analyte mixture using the principles of GC.

GC Detectors
Flame Ionization Detector – produces a small flame from the reaction of hydrogen taken from a tank and oxygen from the air.
Mass Spectrometer Detector – can detect and identify each analyte component as it elutes from the column.
Nitrogen-Phosphorous Detector – it can detect only substances that contain nitrogen or phosphorous. This type is widely used in biological, toxicological, and environmental applications and is very sensitive.
Thermal conductivity Detector – relies on the change in the ability of the mobile phase gas to conduct heat as it is mixed with an analyte. It is simple to engineer and use and is very versatile.
Electron Capture Detector – an extremely sensitive detector that is used on substances that have a halide such as a chloride or bromide, or oxygen in the molecule.
Pyrolysis Gas Chromatography – a modification of GC making it possible for a gas chromatograph to analyze polymers.
Pyrolysis – to heat in the absence of air.
If a polymer such as fiber is heated to very high temperatures, up to 1000°C, in the absence of oxygen, it will not burn but instead will decompose into stable fragments, called pyrolyzates.
Fourier transform infrared spectrophotometer – the GC separates a mixture into individual components and then each one can be introduced into a special sample compartment in an FTIR.
6.5: High-Performance Liquid Chromatography
Stationary phases have become much more efficient in separating components of an analyte, and they are much more sensitive.
The process can be sped up by having the mobile phase pushed through the stationary phase using pumps.
This makes the experiment go much faster while keeping the high resolving power of the technique.
Gradient Chromatography – the composition of the mobile phase can be altered during the run.
Isocratic Chromatography– the mobile phase stays constant during an HPLC run.

HPLC Detectors
Diode array detector – measures the ultraviolet and visible spectrum of the solution as it flows through.
Fluorescence Detector – detect only those substances that exhibit fluorescence, such as the illicit drug LSD. This limits its utility, but it is extremely sensitive. Ultraviolet or visible light is used as the source.
Conductivity detector – detects only conductors.
RI Detector – measures a mobile phase with an analyte dissolved in it will refract light differently than the mobile phase alone.
Mass Spectrometry
When the mobile phase and analytes reach the mass spectrometer, the mobile phase is stripped away and the mass spectrum of each component of the analyte is measured. As in GC, this permits separations and identifications to take place in one step.
Application of HPLC
Drugs
Drugs are more often identified by GC because of the ease with which a mass spectrometer can be used.
Although HPLC/MS instruments are now commercially available, they are not widely used in forensic science laboratories.
Soils
Organic extractions can be done on soils and the various substances can be separated. The result is a profile of the soil.
The substances in the mixture are usually not identified, but the profile is a useful way of determining if soil found at a crime scene could have come from a particular location.
Explosives
It may not be safe to run explosive extracts by GC because of the high heat, but HPLC is an ideal method for the separation of explosive residues.
Inks and dyes:
Determination of the visible and UV spectra of inks is useful in comparing a writing instrument to writing on a document.
It can also be used to follow the aging of the ink as it dries and degrades.
Fiber dyes can be extracted from fibers and separated by HPLC also.
6.6: Thin Layer Chromatography
Paper chromatography is one of the oldest methods of separation and is still used in some applications. The paper acts as a stationary phase and the ink solvent is the mobile phase.
Thin Layer Chromatography — a technique used to isolate non-volatile mixtures.
The experiment is conducted on a sheet of aluminum foil, plastic, or glass which is coated with a thin layer of adsorbent material. The material usually used is aluminum oxide, cellulose, or silica gel.
6.7: Electrophoresis
Two major types of electrophoresis:
Gel electrophoresis – is similar in some respects to TLC; a technique used to separate DNA fragments according to their size.
Capillary electrophoresis – is similar to HPLC; the primary methodology used for separating and detecting short tandem repeat (STR) alleles in forensic DNA laboratories worldwide.
Application of Electrophoresis
DNA Typing
The only method available for separating DNA fragments suitable for forensic DNA typing. Either gel or capillary electrophoresis can be used.
Drugs
Capillary electrophoresis is the most sensitive of the methods.
Explosive Residues
Capillary electrophoresis has been employed because it is more sensitive and has a higher resolution than HPLC. Most organic explosives can be separated by this method.
Gunshot residues
Capillary electrophoresis is very sensitive. It must be optimized for the detection of inorganic substances in this application
Questioned Documents
Capillary electrophoresis has just begun to be used for the separation of ink components used in pens.
 Knowt
Knowt
