
Honors Chemistry A - Unit 6 (Extra Notes/Resources!)
geez louise there’s a lot of stuff this unit, let’s speed through it all
formulas
percent composition
percent composition is just the percentage of a certain element in a compound

it’s like saying “oh yah that compound is 20% iron” or “bella’s diet is 95% smarties”
steps:
find mass of the element ur finding the percent of (and don’t forget to multiply by number of atoms there are, or if its a BrINClHOF element)
find total mass of compound
plug into the formula! divide the mass of the element by the total mass of the compound, then multiply by 100 to get a percentage!
vid: https://www.youtube.com/watch?v=JqKEaPmNFLg&list=PLytGGifnD9dUom302biLWWGuTxg7lgI7P&index=11
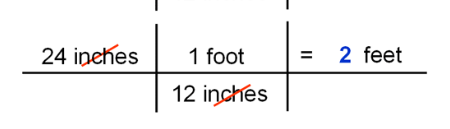
dimensional analysis
dimensional analysis is using fractions to turn one unit of measurement to another
you take your starting unit, then multiply it by another fraction with the new unit of measurement on top, and the one you want to cancel out on the bottom
find empirical formula from % composition
example problem: A compound is 71.65% chlorine, 24.27% carbon, and 4.07% hydrogen. What is the empirical formula of the compound?
Convert %’s into grams (if the question already gives the info in grams, skip this step)
71.65% Cl → 71.65 g Cl
24.27% C → 24.27 g C
4.07% H → 4.07 g H
Convert grams to moles
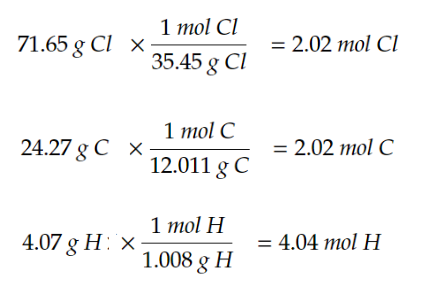
now divide alll the numbers by the the smallest number of moles
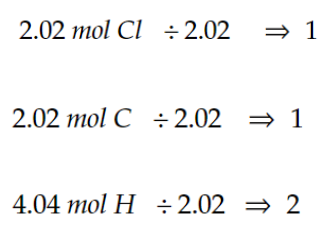
if the numbers aren’t whole, multiply them so they’re whole, we don’t do decimals here
and voila, the numbers u got are now the subscripts for the elements!
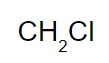
find molecular formula from empirical formula
we’re gonna go off the last question from above^, cuz that’s how it’ll be formatted on the test
so: If the molar mass of the previous compound (CH2Cl) is 148.41 g, what is the molecular formula?
find total mass of empirical formula

divide molar mass by empirical mass
148.41 / 49.48 = 3
take that number ^, and multiply all the subscripts of the empirical formula by it, and bada bam boom, you have molecular formula

tidbits u shd know
atomic mass = 1 mol of an element
molar mass= atomic mass = atomic weight = molecular weight
amu = g/mol
OMG RESOURCES!!!
percent composition → http://rushwjhs.weebly.com/uploads/5/4/5/4/54543371/percent_composition_practice_wrksht_ii.pdf
moles/dimensional analysis → https://www.everettcc.edu/files/programs/academic-resources/transitional-studies/support/tutoring-center/chemistry/w340-mole-calculations-worksheet-1.pdf
empirical formula →https://www.thoughtco.com/empirical-formula-practice-test-questions-604118
molecular/empirical formula → https://docs.google.com/document/d/1ps06WerffLaVhGcFspQIfhsQ9xKNq-XZecAIgd9XwX8/edit?usp=sharing
Honors Chemistry A - Unit 6 (Extra Notes/Resources!)
geez louise there’s a lot of stuff this unit, let’s speed through it all
formulas
percent composition
percent composition is just the percentage of a certain element in a compound

it’s like saying “oh yah that compound is 20% iron” or “bella’s diet is 95% smarties”
steps:
find mass of the element ur finding the percent of (and don’t forget to multiply by number of atoms there are, or if its a BrINClHOF element)
find total mass of compound
plug into the formula! divide the mass of the element by the total mass of the compound, then multiply by 100 to get a percentage!
vid: https://www.youtube.com/watch?v=JqKEaPmNFLg&list=PLytGGifnD9dUom302biLWWGuTxg7lgI7P&index=11
dimensional analysis
dimensional analysis is using fractions to turn one unit of measurement to another
you take your starting unit, then multiply it by another fraction with the new unit of measurement on top, and the one you want to cancel out on the bottom

find empirical formula from % composition
example problem: A compound is 71.65% chlorine, 24.27% carbon, and 4.07% hydrogen. What is the empirical formula of the compound?
Convert %’s into grams (if the question already gives the info in grams, skip this step)
71.65% Cl → 71.65 g Cl
24.27% C → 24.27 g C
4.07% H → 4.07 g H
Convert grams to moles
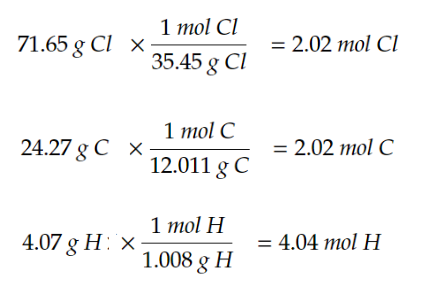
now divide alll the numbers by the the smallest number of moles
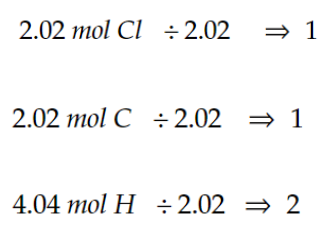
if the numbers aren’t whole, multiply them so they’re whole, we don’t do decimals here
and voila, the numbers u got are now the subscripts for the elements!
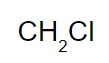
find molecular formula from empirical formula
we’re gonna go off the last question from above^, cuz that’s how it’ll be formatted on the test
so: If the molar mass of the previous compound (CH2Cl) is 148.41 g, what is the molecular formula?
find total mass of empirical formula

divide molar mass by empirical mass
148.41 / 49.48 = 3
take that number ^, and multiply all the subscripts of the empirical formula by it, and bada bam boom, you have molecular formula

tidbits u shd know
atomic mass = 1 mol of an element
molar mass= atomic mass = atomic weight = molecular weight
amu = g/mol
OMG RESOURCES!!!
percent composition → http://rushwjhs.weebly.com/uploads/5/4/5/4/54543371/percent_composition_practice_wrksht_ii.pdf
moles/dimensional analysis → https://www.everettcc.edu/files/programs/academic-resources/transitional-studies/support/tutoring-center/chemistry/w340-mole-calculations-worksheet-1.pdf
empirical formula →https://www.thoughtco.com/empirical-formula-practice-test-questions-604118
molecular/empirical formula → https://docs.google.com/document/d/1ps06WerffLaVhGcFspQIfhsQ9xKNq-XZecAIgd9XwX8/edit?usp=sharing
 Knowt
Knowt