
Chapter 15: Lipids
15.1: Lipids
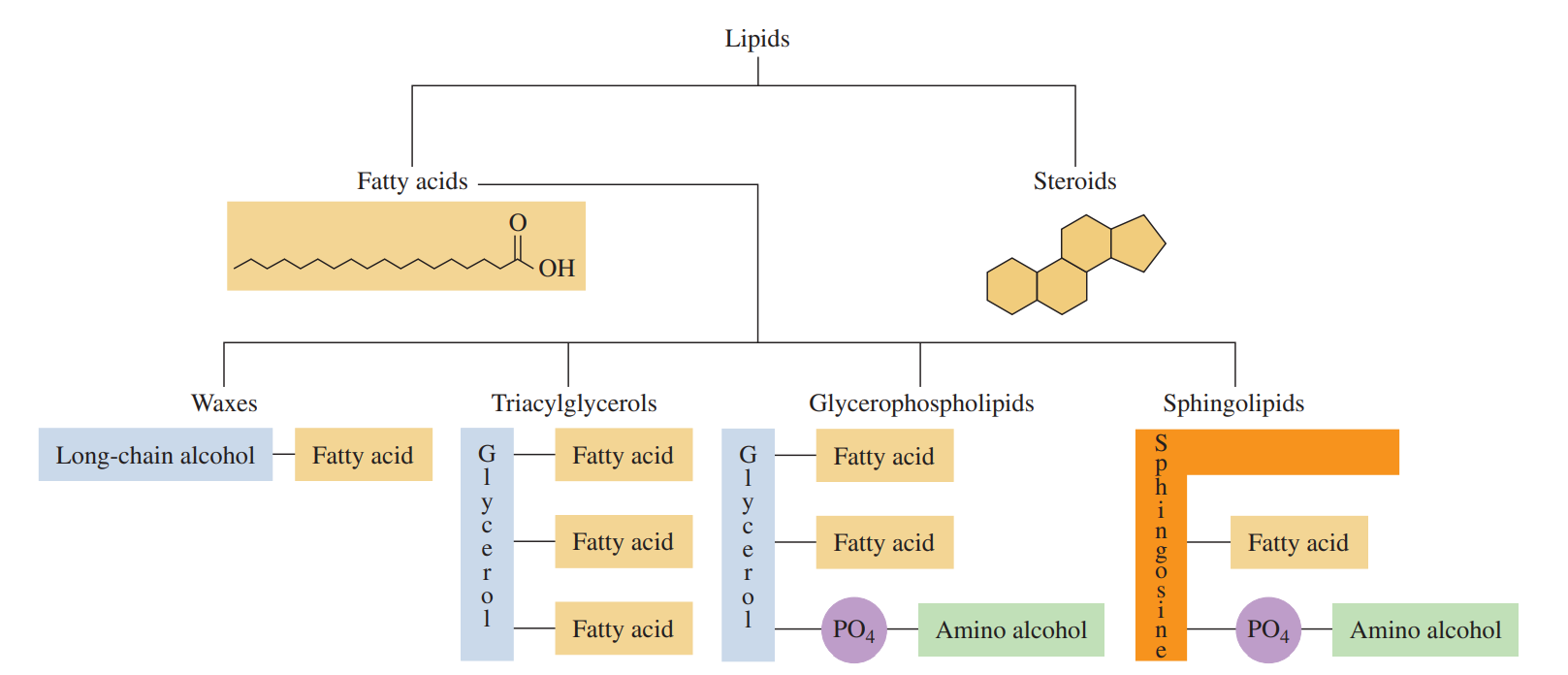
Lipids: These are a family of biomolecules that have the common property of being soluble in organic solvents but not in water.
The lipid content of a cell can be extracted using a nonpolar solvent such as ether or chloroform.
Lipids such as waxes, fats, oils, and phospholipids are esters that can be hydrolyzed to give fatty acids along with other molecules.
Steroids are characterized by the steroid nucleus of four fused carbon rings.
They do not contain fatty acids and cannot be hydrolyzed.
15.2: Fatty Acids
Fatty Acid (CH3(CH2)nCOOH): It contains a long, unbranched carbon chain with a carboxylic acid group at one end.
Saturated Fatty Acid: It contains only carbon–carbon single bonds, which make the properties of a long-chain fatty acid similar to those of an alkane.
Unsaturated Fatty Acid: It contains one or more carbon–carbon double bonds.
It can be drawn as cis and trans isomers.
Monosaturated Fatty Acid: The long carbon chain has one double bond, which makes its properties similar to those of an alkene.
Polyunsaturated Fatty Acid: It has at least two carbon–carbon double bonds.
Prostaglandins: These are hormone-like substances produced in small amounts in most cells of the body.
These are also known as eicosanoids; which are formed from arachidonic acid, the polyunsaturated fatty acid with 20 carbon atoms.
Structures and Melting Points of Common Fatty Acids

15.3: Waxes and Triacylglycerol
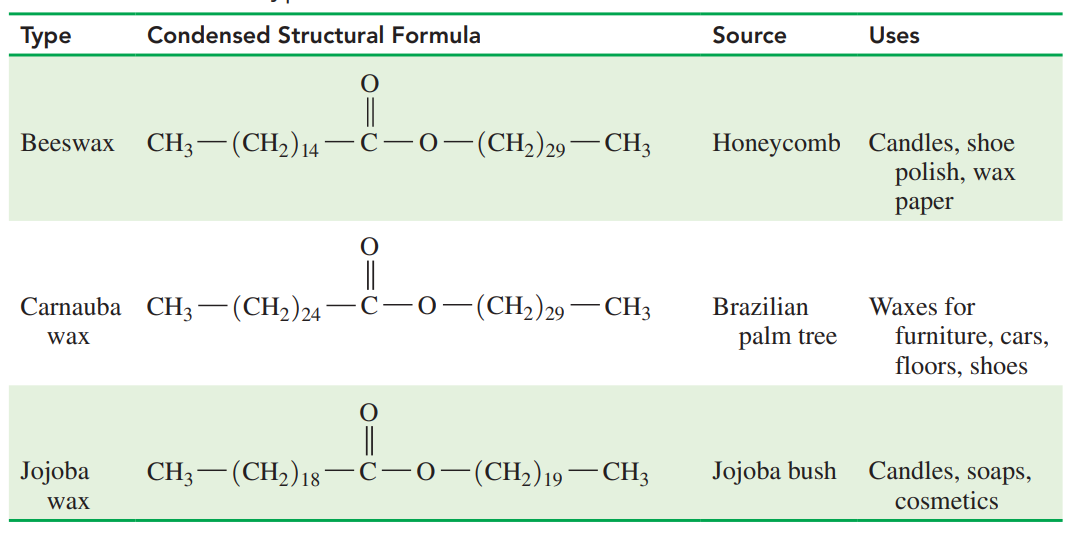
Wax: An ester of a saturated fatty acid and a long-chain alcohol, each containing from 14 to 30 carbon atoms.
Waxes are found in many plants and animals.
Natural waxes are found on the surface of fruits, and on the leaves and stems of plants where they help prevent loss of water and damage from pests.
Waxes on the skin, fur, and feathers of animals provide a waterproof coating.
Triacylglycerols (C66H110O6): This is where fatty acids are stored, which are triesters of glycerol and fatty acids
Most naturally occurring triacylglycerols contain glycerol bonded to two or three different fatty acids, typically palmitic acid, oleic acid, linoleic acid, and stearic acid.
Triacylglycerols are the major form of energy storage for animals.
Fat: A triacylglycerol solid at room temperature, and it usually comes from animal sources such as meat, whole milk, butter, and cheese.
Oil: A triacylglycerol is usually a liquid at room temperature and is obtained from a plant source.
15.4: Chemical Properties of Triaglyceroles
Hydrogenation
In this reaction, hydrogen gas is bubbled through the heated oil typically in the presence of a nickel catalyst.
H atoms add to one or more carbon–carbon double bonds to form carbon–carbon single bonds.
Hydrolysis
Triacylglycerols are hydrolyzed in the presence of strong acids or lipases.
The products of hydrolysis of the ester bonds are glycerol and three fatty acids.
Polar glycerol is soluble in water, but the fatty acids with their long hydrocarbon chains are not.
Saponification
It occurs when fat is heated with a strong base.
When NaOH is used, a solid soap is produced that can be molded into a desired shape; KOH produces a softer, liquid soap.
An oil that is polyunsaturated produces a softer soap.
15.5: Phospholipids
Phospholipids: These are a family of lipids similar in structure to triacylglycerols; they include glycerophospholipids and sphingomyelins.
In glycerophospholipid, two fatty acids form ester bonds with the first and second hydroxyl groups of glycerol.
In sphingomyelin, sphingosine replaces glycerol.
The amine group of sphingosine forms an amide bond to a fatty acid and the hydroxyl group forms an ester bond with phosphate, which forms another phosphodiester bond to choline or ethanolamine.
Lecithins (C35H66NO7P) and cephalins (C9H18NO8P) are two types of glycerophospholipids that are particularly abundant in brain and nerve tissues as well as in egg yolks, wheat germ, and yeast.
Glycerophospholipids contain both polar and nonpolar regions, which allow them to interact with both polar and nonpolar substances.
Snake venom is produced by the modified saliva glands of poisonous snakes. When a snake bites, venom is ejected through the fang of the snake.
The venom of the eastern diamondback rattlesnake and the Indian cobra contains phospholipase (C106H184N28O30), which are enzymes that catalyze the hydrolysis of the fatty acid on the center carbon of glycerophospholipids in the red blood cells.
Lysophospholipid causes the breakdown of the red blood cell membranes. This makes them permeable to water, which causes hemolysis of the red blood cells.
15.6: Steroids
Steroids (C19H28O2)
These are compounds containing the steroid nucleus, which consists of three cyclohexane rings and one cyclopentane ring fused together.
The four rings in the steroid nucleus are designated A, B, C, and D.
Cholesterol (C27H46O)
It is one of the most important and abundant steroids in the body, is a sterol because it contains an oxygen atom as a hydroxyl group on carbon 3.
It is a component of cellular membranes, myelin sheath, and brain and nerve tissue.
It is also found in the liver and bile salts.
In the adrenal gland, cholesterol is used to synthesize steroid hormones.
Bile Salts (C24H40O5**)**
These are synthesized from cholesterol in the liver and stored in the gallbladder.
When bile is secreted into the small intestine, the bile salts mix with the water-insoluble fats and oils in our diets.
The bile salts with their nonpolar and polar regions act much like soaps, breaking down large globules of fat into smaller droplets.
The smaller droplets containing fat have a larger surface area to react with lipases, which are the enzymes that digest fat.
**Lipoproteins (**C27H46O): They are made more soluble by combining them with phospholipids and proteins to form water-soluble complexes.
Steroid Hormones
Hormones: These are chemical messengers that serve as a communication system from one part of the body to another
Steroid Hormones: These are closely related in structure to cholesterol and depend on cholesterol for their synthesis (sex hormones and adrenocortical hormones).
Two of the male sex hormones: testosterone and androsterone, promote the growth of muscle and facial hair, and the maturation of the male sex organs and of sperm.
Estrogens: A group of female sex hormones, that direct the development of female sexual characteristics.
Progesterone: It prepares the uterus for the implantation of a fertilized egg.
Adrenal Corticosteroids
Corticosteroids: Produced by the adrenal glands.
Cortisone: It increases the blood glucose level and stimulates the synthesis of glycogen in the liver.
Aldosterone: It is responsible for the regulation of electrolytes and water balance by the kidneys.
Cortisol: It is released under stress to increase blood sugar and regulate carbohydrate, fat, and protein metabolism.
Prednisone: These are synthetic corticosteroid drugs that are derived from cortisone and used medically for reducing inflammation and treating asthma and rheumatoid arthritis, although health problems can result from long-term use.
15.7: Cell Membranes
The membrane of a cell separates the contents of a cell from the external fluids.
It is semi-permeable so that nutrients can enter the cell and waste products can leave.
The main components of a cell membrane are the glycerophospholipids and sphingolipids.
Lipid Bilayer: The double-layer arrangement of phospholipids.
The outer layer of phospholipids is in contact with the external fluids.
The inner layer is in contact with the internal contents of the cell.
The model of biological membranes is referred to as the fluid mosaic model of membranes.
In the fluid mosaic model, proteins known as peripheral proteins emerge on just one of the surfaces, outer or inner.
The integral proteins extend through the entire lipid bilayer and appear on both surfaces of the membrane.

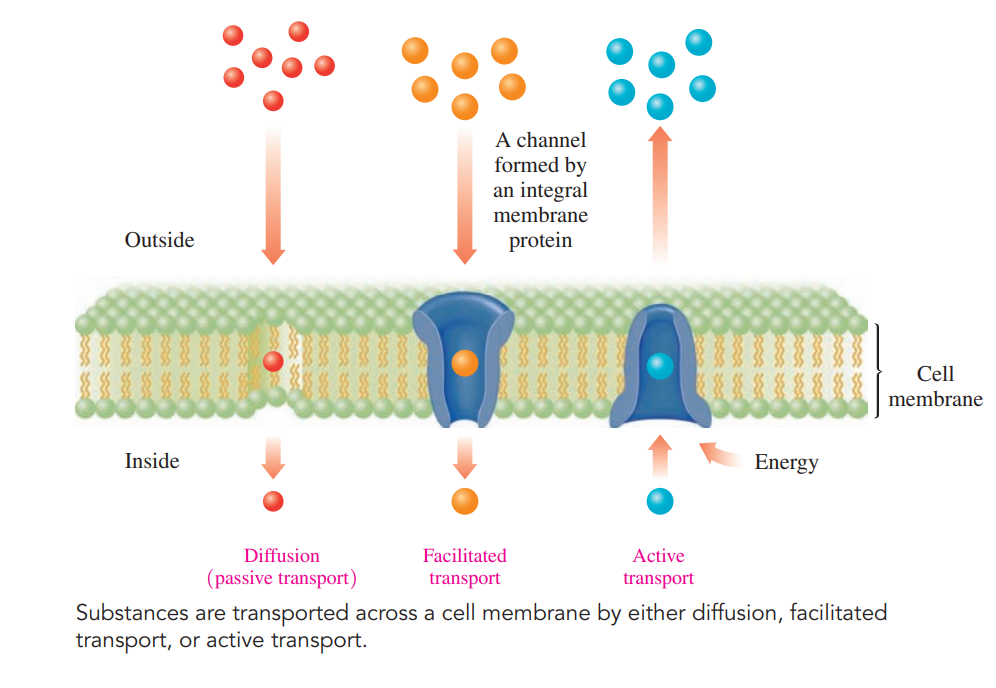
Transport through Cell Membranes
Diffusion Transport: Molecules can diffuse from a higher concentration to a lower concentration.
Facilitated Transport: Proteins that extend from one side of the bilayer membrane to the other provide a channel through which certain substances can diffuse more rapidly than by passive diffusion to meet cellular needs.
Active Transport
Certain ions such as K+, Na +, and Ca2+ move across a cell membrane against their concentration gradients.
The K+ concentration is greater inside a cell, and the Na + concentration is greater outside.
However, in the conduction of nerve impulses and contraction of muscles, K+ moves into the cell, and Na + moves out by active transport.
Chapter 15: Lipids
15.1: Lipids
Lipids: These are a family of biomolecules that have the common property of being soluble in organic solvents but not in water.
The lipid content of a cell can be extracted using a nonpolar solvent such as ether or chloroform.
Lipids such as waxes, fats, oils, and phospholipids are esters that can be hydrolyzed to give fatty acids along with other molecules.
Steroids are characterized by the steroid nucleus of four fused carbon rings.
They do not contain fatty acids and cannot be hydrolyzed.

15.2: Fatty Acids
Fatty Acid (CH3(CH2)nCOOH): It contains a long, unbranched carbon chain with a carboxylic acid group at one end.
Saturated Fatty Acid: It contains only carbon–carbon single bonds, which make the properties of a long-chain fatty acid similar to those of an alkane.
Unsaturated Fatty Acid: It contains one or more carbon–carbon double bonds.
It can be drawn as cis and trans isomers.
Monosaturated Fatty Acid: The long carbon chain has one double bond, which makes its properties similar to those of an alkene.
Polyunsaturated Fatty Acid: It has at least two carbon–carbon double bonds.
Prostaglandins: These are hormone-like substances produced in small amounts in most cells of the body.
These are also known as eicosanoids; which are formed from arachidonic acid, the polyunsaturated fatty acid with 20 carbon atoms.
Structures and Melting Points of Common Fatty Acids

15.3: Waxes and Triacylglycerol
Wax: An ester of a saturated fatty acid and a long-chain alcohol, each containing from 14 to 30 carbon atoms.
Waxes are found in many plants and animals.
Natural waxes are found on the surface of fruits, and on the leaves and stems of plants where they help prevent loss of water and damage from pests.
Waxes on the skin, fur, and feathers of animals provide a waterproof coating.

Triacylglycerols (C66H110O6): This is where fatty acids are stored, which are triesters of glycerol and fatty acids
Most naturally occurring triacylglycerols contain glycerol bonded to two or three different fatty acids, typically palmitic acid, oleic acid, linoleic acid, and stearic acid.
Triacylglycerols are the major form of energy storage for animals.
Fat: A triacylglycerol solid at room temperature, and it usually comes from animal sources such as meat, whole milk, butter, and cheese.
Oil: A triacylglycerol is usually a liquid at room temperature and is obtained from a plant source.
15.4: Chemical Properties of Triaglyceroles
Hydrogenation
In this reaction, hydrogen gas is bubbled through the heated oil typically in the presence of a nickel catalyst.
H atoms add to one or more carbon–carbon double bonds to form carbon–carbon single bonds.
Hydrolysis
Triacylglycerols are hydrolyzed in the presence of strong acids or lipases.
The products of hydrolysis of the ester bonds are glycerol and three fatty acids.
Polar glycerol is soluble in water, but the fatty acids with their long hydrocarbon chains are not.
Saponification
It occurs when fat is heated with a strong base.
When NaOH is used, a solid soap is produced that can be molded into a desired shape; KOH produces a softer, liquid soap.
An oil that is polyunsaturated produces a softer soap.
15.5: Phospholipids
Phospholipids: These are a family of lipids similar in structure to triacylglycerols; they include glycerophospholipids and sphingomyelins.
In glycerophospholipid, two fatty acids form ester bonds with the first and second hydroxyl groups of glycerol.
In sphingomyelin, sphingosine replaces glycerol.
The amine group of sphingosine forms an amide bond to a fatty acid and the hydroxyl group forms an ester bond with phosphate, which forms another phosphodiester bond to choline or ethanolamine.
Lecithins (C35H66NO7P) and cephalins (C9H18NO8P) are two types of glycerophospholipids that are particularly abundant in brain and nerve tissues as well as in egg yolks, wheat germ, and yeast.
Glycerophospholipids contain both polar and nonpolar regions, which allow them to interact with both polar and nonpolar substances.
Snake venom is produced by the modified saliva glands of poisonous snakes. When a snake bites, venom is ejected through the fang of the snake.
The venom of the eastern diamondback rattlesnake and the Indian cobra contains phospholipase (C106H184N28O30), which are enzymes that catalyze the hydrolysis of the fatty acid on the center carbon of glycerophospholipids in the red blood cells.
Lysophospholipid causes the breakdown of the red blood cell membranes. This makes them permeable to water, which causes hemolysis of the red blood cells.
15.6: Steroids
Steroids (C19H28O2)
These are compounds containing the steroid nucleus, which consists of three cyclohexane rings and one cyclopentane ring fused together.
The four rings in the steroid nucleus are designated A, B, C, and D.
Cholesterol (C27H46O)
It is one of the most important and abundant steroids in the body, is a sterol because it contains an oxygen atom as a hydroxyl group on carbon 3.
It is a component of cellular membranes, myelin sheath, and brain and nerve tissue.
It is also found in the liver and bile salts.
In the adrenal gland, cholesterol is used to synthesize steroid hormones.
Bile Salts (C24H40O5**)**
These are synthesized from cholesterol in the liver and stored in the gallbladder.
When bile is secreted into the small intestine, the bile salts mix with the water-insoluble fats and oils in our diets.
The bile salts with their nonpolar and polar regions act much like soaps, breaking down large globules of fat into smaller droplets.
The smaller droplets containing fat have a larger surface area to react with lipases, which are the enzymes that digest fat.
**Lipoproteins (**C27H46O): They are made more soluble by combining them with phospholipids and proteins to form water-soluble complexes.
Steroid Hormones
Hormones: These are chemical messengers that serve as a communication system from one part of the body to another
Steroid Hormones: These are closely related in structure to cholesterol and depend on cholesterol for their synthesis (sex hormones and adrenocortical hormones).
Two of the male sex hormones: testosterone and androsterone, promote the growth of muscle and facial hair, and the maturation of the male sex organs and of sperm.
Estrogens: A group of female sex hormones, that direct the development of female sexual characteristics.
Progesterone: It prepares the uterus for the implantation of a fertilized egg.
Adrenal Corticosteroids
Corticosteroids: Produced by the adrenal glands.
Cortisone: It increases the blood glucose level and stimulates the synthesis of glycogen in the liver.
Aldosterone: It is responsible for the regulation of electrolytes and water balance by the kidneys.
Cortisol: It is released under stress to increase blood sugar and regulate carbohydrate, fat, and protein metabolism.
Prednisone: These are synthetic corticosteroid drugs that are derived from cortisone and used medically for reducing inflammation and treating asthma and rheumatoid arthritis, although health problems can result from long-term use.
15.7: Cell Membranes
The membrane of a cell separates the contents of a cell from the external fluids.
It is semi-permeable so that nutrients can enter the cell and waste products can leave.
The main components of a cell membrane are the glycerophospholipids and sphingolipids.
Lipid Bilayer: The double-layer arrangement of phospholipids.
The outer layer of phospholipids is in contact with the external fluids.
The inner layer is in contact with the internal contents of the cell.
The model of biological membranes is referred to as the fluid mosaic model of membranes.
In the fluid mosaic model, proteins known as peripheral proteins emerge on just one of the surfaces, outer or inner.
The integral proteins extend through the entire lipid bilayer and appear on both surfaces of the membrane.

Transport through Cell Membranes
Diffusion Transport: Molecules can diffuse from a higher concentration to a lower concentration.
Facilitated Transport: Proteins that extend from one side of the bilayer membrane to the other provide a channel through which certain substances can diffuse more rapidly than by passive diffusion to meet cellular needs.
Active Transport
Certain ions such as K+, Na +, and Ca2+ move across a cell membrane against their concentration gradients.
The K+ concentration is greater inside a cell, and the Na + concentration is greater outside.
However, in the conduction of nerve impulses and contraction of muscles, K+ moves into the cell, and Na + moves out by active transport.

 Knowt
Knowt
