
16 Sulfur
Sulfur sources and uses
Sulfur is found in its elemental state underground in the USA, Mexico and Poland
It is also a by-product from the removal of sulfur from petroleum and natural gas
Sulfur can also be obtained from sulfide ores
The main use of sulfur is in making sulphuric acid which is a very important chemical used in many industries
It is also used extensively in making rubber tyres more flexible (vulcanising), where the rubber is heated with sulfur
Sulfur dioxide
Sulfur dioxide can be made by burning sulphur in air
This is the method used in the first stage of the manufacture of sulfuric acid
Sulfur dioxide is used as a bleach in the manufacture of wood pulp for paper, and as a preservative for foods and drinks by killing bacteria
Sulfites are often added to foods and these release sulfur dioxide in acidic conditions

Sulfuric Acid : Properties, Manufacture and uses
Sulfuric acid is synthesised by the Contact process which use sulfur and oxygen from air and is done in three distinct stages
The Contact Process
Stage 1
The first stage is the oxidation of sulfur:
S + O2 → SO2
Stage 2
The main stage is the oxidation of sulfur dioxide to sulfur trioxide using a V2O5 catalyst:

The conditions for the main stage of production of sulfur trioxide need to be considered
Conditions during Stage 2
Temperature 450°
The reaction is exothermic, so increasing the temperature shifts the position of equilibrium to the left in the direction of the reactants
Therefore the higher the temperature, the lower the yield of sulfur trioxide
The optimum temperature is a compromise between a higher rate of reaction at a higher temperature and a lower equilibrium yield at a higher temperature
Pressure: 2 atm
An increase in pressure shifts the position of equilibrium to the right in the direction of a smaller number of gaseous molecules
However the position of equilibrium lies far to the right (the equilibrium mixture contains about 96% sulfur trioxide)
So the reaction is carried out at just above atmospheric pressure because:
it is not worth spending the extra energy or money required to produce high pressures
a higher pressure causes the sulfur dioxide to liquefy
Stage 3
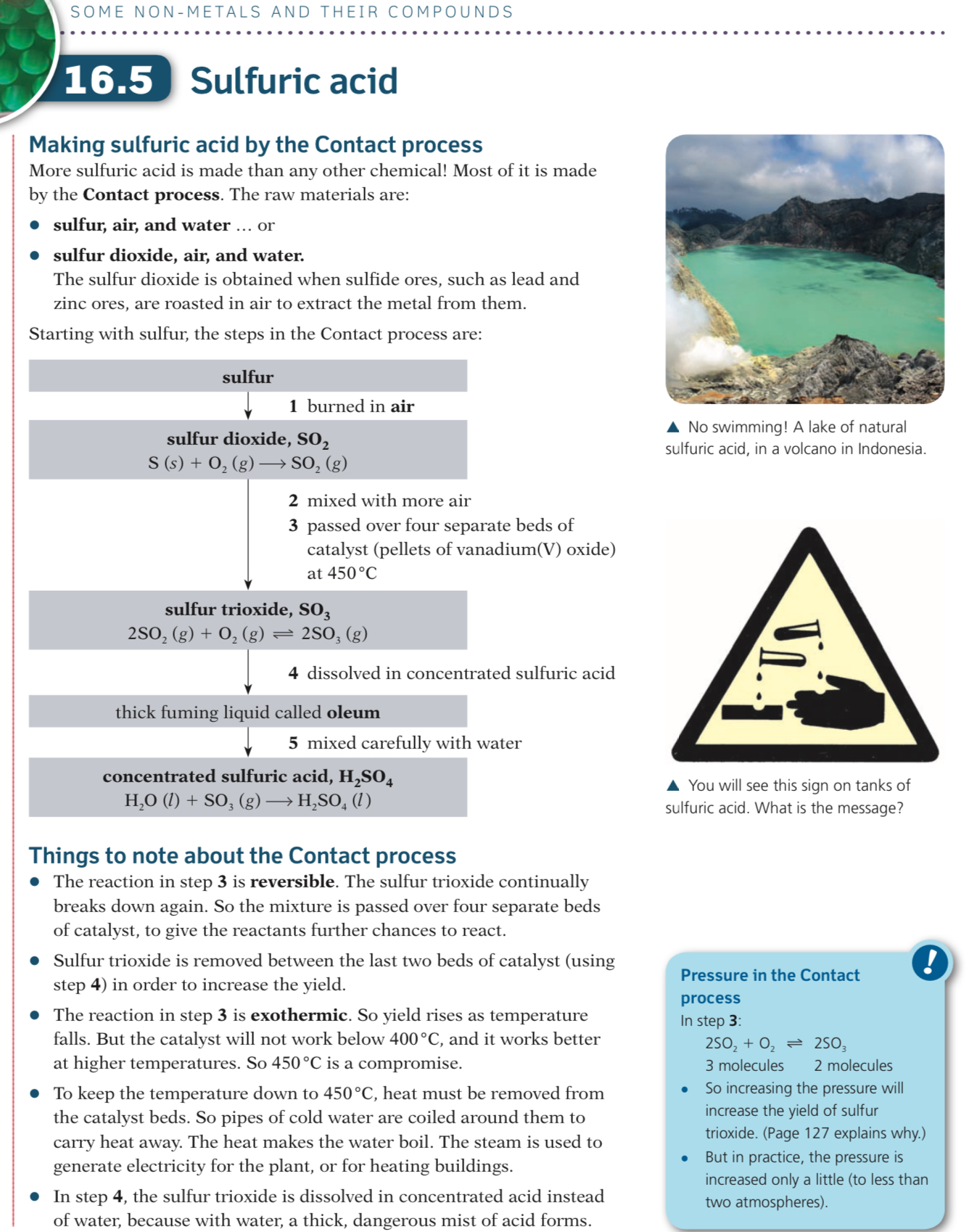
Once stage 2 is completed, the sulfur trioxide is absorbed into a solution of 98% sulphuric acid to produce a thick liquid called oleum:
SO3 + H2SO4 → H2SO7
It is not absorbed into water because a fine mist of sulfuric acid would be produced and this would be difficult to condense and is also highly dangerous
Oleum is added to water to form concentrated sulfuric acid:
Properties of sulfuric acid
Sulfuric acid is a strong dibasic acid as two of its hydrogen atoms can be replaced by a metal
Mg + H2SO4 → MgSO4 + H2
It reacts in a similar way to other acids with metal carbonates, oxides, hydroxides (and ammonia) and metals, e.g:
Na2CO3 + H2SO4 → Na2SO4 + CO2+ H2O
ZnO + H2SO4 → ZnSO4 + H2O
Concentrated sulphuric acid is corrosive and a powerful oxidising agent
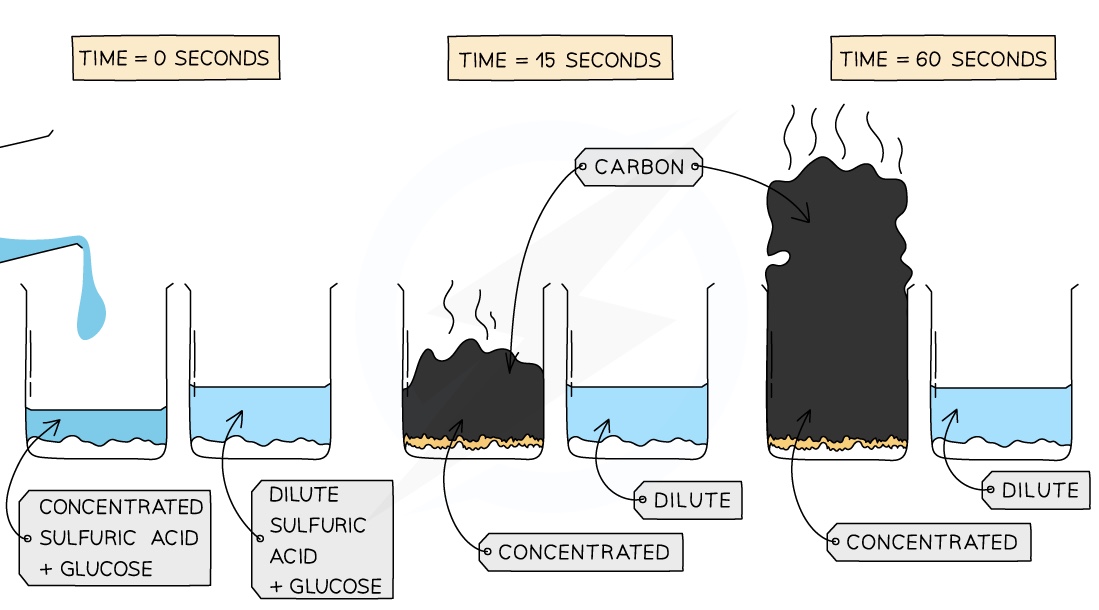
Concentrated sulphuric acid is also a very powerful dehydrating agent and is very good at removing water from other substances
For example, if mixed with sugar (C6H12O6), concentrated H2SO4 will remove water molecules and leave behind carbon in a spectacular looking black tower
Uses of Sulfuric Acid
In dilute solution it is used as a catalyst in many organic reactions and to clean the surface of metals
Concentrated sulfuric acid is used in car batteries, making fertilisers, soaps and detergents
It is also used to make acid drain cleaners and in the production of paints and dyes

16 Sulfur
Sulfur sources and uses
Sulfur is found in its elemental state underground in the USA, Mexico and Poland
It is also a by-product from the removal of sulfur from petroleum and natural gas
Sulfur can also be obtained from sulfide ores
The main use of sulfur is in making sulphuric acid which is a very important chemical used in many industries
It is also used extensively in making rubber tyres more flexible (vulcanising), where the rubber is heated with sulfur
Sulfur dioxide
Sulfur dioxide can be made by burning sulphur in air
This is the method used in the first stage of the manufacture of sulfuric acid
Sulfur dioxide is used as a bleach in the manufacture of wood pulp for paper, and as a preservative for foods and drinks by killing bacteria
Sulfites are often added to foods and these release sulfur dioxide in acidic conditions


Sulfuric Acid : Properties, Manufacture and uses

Sulfuric acid is synthesised by the Contact process which use sulfur and oxygen from air and is done in three distinct stages
The Contact Process
Stage 1
The first stage is the oxidation of sulfur:
S + O2 → SO2
Stage 2
The main stage is the oxidation of sulfur dioxide to sulfur trioxide using a V2O5 catalyst:

The conditions for the main stage of production of sulfur trioxide need to be considered
Conditions during Stage 2
Temperature 450°
The reaction is exothermic, so increasing the temperature shifts the position of equilibrium to the left in the direction of the reactants
Therefore the higher the temperature, the lower the yield of sulfur trioxide
The optimum temperature is a compromise between a higher rate of reaction at a higher temperature and a lower equilibrium yield at a higher temperature
Pressure: 2 atm
An increase in pressure shifts the position of equilibrium to the right in the direction of a smaller number of gaseous molecules
However the position of equilibrium lies far to the right (the equilibrium mixture contains about 96% sulfur trioxide)
So the reaction is carried out at just above atmospheric pressure because:
it is not worth spending the extra energy or money required to produce high pressures
a higher pressure causes the sulfur dioxide to liquefy
Stage 3
Once stage 2 is completed, the sulfur trioxide is absorbed into a solution of 98% sulphuric acid to produce a thick liquid called oleum:
SO3 + H2SO4 → H2SO7
It is not absorbed into water because a fine mist of sulfuric acid would be produced and this would be difficult to condense and is also highly dangerous
Oleum is added to water to form concentrated sulfuric acid:
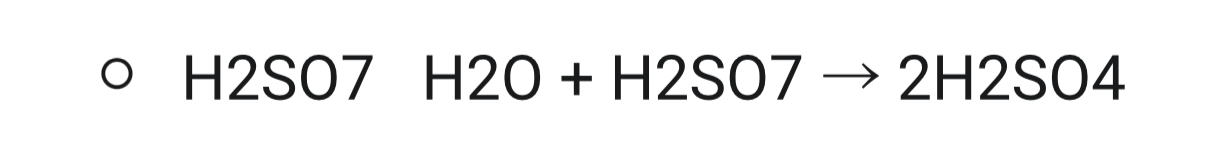
Properties of sulfuric acid
Sulfuric acid is a strong dibasic acid as two of its hydrogen atoms can be replaced by a metal
Mg + H2SO4 → MgSO4 + H2
It reacts in a similar way to other acids with metal carbonates, oxides, hydroxides (and ammonia) and metals, e.g:
Na2CO3 + H2SO4 → Na2SO4 + CO2+ H2O
ZnO + H2SO4 → ZnSO4 + H2O
Concentrated sulphuric acid is corrosive and a powerful oxidising agent
Concentrated sulphuric acid is also a very powerful dehydrating agent and is very good at removing water from other substances
For example, if mixed with sugar (C6H12O6), concentrated H2SO4 will remove water molecules and leave behind carbon in a spectacular looking black tower

Uses of Sulfuric Acid
In dilute solution it is used as a catalyst in many organic reactions and to clean the surface of metals
Concentrated sulfuric acid is used in car batteries, making fertilisers, soaps and detergents
It is also used to make acid drain cleaners and in the production of paints and dyes

 Knowt
Knowt
