
Chapter 3 - Enzymes
Enzymes
Enzymes are biological catalysts that speed up the rate of chemical reactions without being altered in the reaction. They are made of proteins.
Enzymes work by lowering the activation energy of a chemical reaction. Activation energy is the amount of energy needed for a reaction to take place.
Enzymes allow biochemical reactions to take place without drastic conditions such as high temperatures because less heat energy is required to start a reaction.
Enzymes can break down or build up biological molecules.
Enzymes are required in small amounts because they %%remain unchanged %%in the chemical reactions they catalyse and can be reused.
They are substrate-specific. Substrates are the reactants that an enzyme acts on. Each enzyme can only act on the particular substrate of the reaction they are supposed to catalyse. For example, amylase can only digest starch and not cellulose even though they are both polymers of glucose.
Therefore, each enzyme catalyses a different reaction. This is due to its unique 3-dimensional structure.
Lock and Key Hypothesis:
The ‘lock and key’ hypothesis relates enzyme specificity to the presence of active sites. An active site is the region on an enzyme molecule that the substrate binds to. It is usually a pocket or groove on the surface of the enzyme that is part of the enzyme’s unique 3-dimensional structure.
The shape of the active site conforms to the substrate. Only the correct substrate is able to fit into the active site.
The process begins when the substrate molecule binds to the active site of the enzyme to form an enzyme-substrate complex.
The reaction is then catalysed at the active sites to convert the substrate into product molecules.
The product molecules depart from the active site, leaving the enzyme free to catalyse another reaction.
The diagram below illustrates the ‘lock and key’ hypothesis for a reaction in which an enzyme breaks down a substrate molecule into 2 product molecules:
![]()
Effect of Temperature on the rate of reaction:
The effects of temperature on the rate of enzyme-catalysed reactions is shown in the graph below:
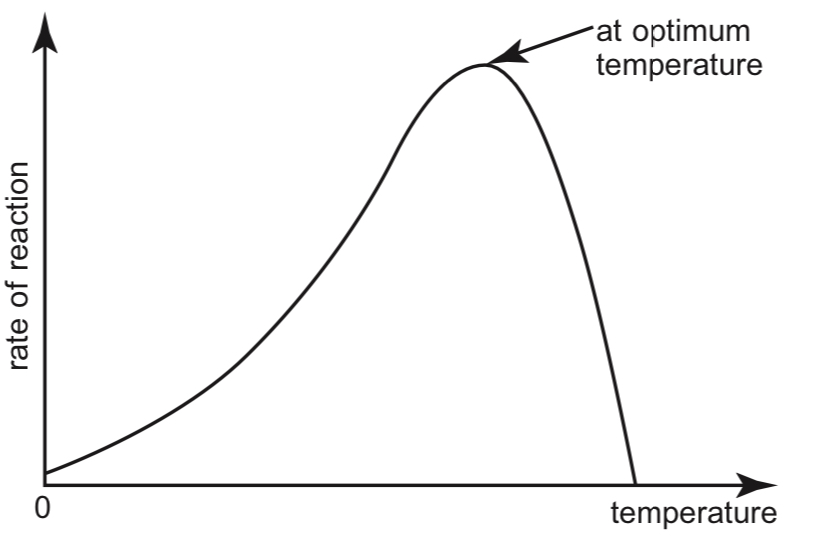
At low temperatures, enzymes are inactive and the rate of reaction is very low. Substrate and enzyme molecules have little kinetic energy, hence the frequency of collision is low. In addition, most substrate molecules do not contain sufficient energy to overcome the activation energy required to start a reaction.
As temperature increases, the rate of enzyme activity increases. Enzyme activity doubles with every 10°C rise in temperature. This is because the reactants have higher levels of energy, and the substrate molecules are able to collide with active sites more frequently.
At the optimum temperature, enzyme activity is the highest.
As the temperature increases beyond the optimum temperature, enzyme activity drops sharply. This is because enzymes are made of proteins, which are denatured at high temperatures. The enzyme loses its 3-dimensional structure and active site conformation due to the breaking of the weak bonds that hold the structure together.
At extremely high temperatures, the enzyme is completely denatured and the rate of reaction drops to zero.
Effect of pH on the rate of reaction:
The graph showing the effects of pH on the rate of enzyme-catalysed reactions is shown in the graph below:
![]()
Enzyme activity is the highest at the optimum pH of the enzyme.
As the pH increases or decreases from the optimum, enzyme activity sharply decreases. This is because the hydrogen bonds and ionic bonds that hold the 3-dimensional structure are disrupted. The shape of the active site is changed as the enzyme is denatured.
At extreme pH levels, the enzyme is completely denatured and the rate of reaction drops to zero.
The optimum pH for each enzyme differs. For example, pepsin works best under the acidic conditions in the stomach, while intestinal enzymes work best under alkaline conditions.
Chapter 3 - Enzymes
Enzymes
Enzymes are biological catalysts that speed up the rate of chemical reactions without being altered in the reaction. They are made of proteins.
Enzymes work by lowering the activation energy of a chemical reaction. Activation energy is the amount of energy needed for a reaction to take place.
Enzymes allow biochemical reactions to take place without drastic conditions such as high temperatures because less heat energy is required to start a reaction.
Enzymes can break down or build up biological molecules.
Enzymes are required in small amounts because they %%remain unchanged %%in the chemical reactions they catalyse and can be reused.
They are substrate-specific. Substrates are the reactants that an enzyme acts on. Each enzyme can only act on the particular substrate of the reaction they are supposed to catalyse. For example, amylase can only digest starch and not cellulose even though they are both polymers of glucose.
Therefore, each enzyme catalyses a different reaction. This is due to its unique 3-dimensional structure.
Lock and Key Hypothesis:
The ‘lock and key’ hypothesis relates enzyme specificity to the presence of active sites. An active site is the region on an enzyme molecule that the substrate binds to. It is usually a pocket or groove on the surface of the enzyme that is part of the enzyme’s unique 3-dimensional structure.
The shape of the active site conforms to the substrate. Only the correct substrate is able to fit into the active site.
The process begins when the substrate molecule binds to the active site of the enzyme to form an enzyme-substrate complex.
The reaction is then catalysed at the active sites to convert the substrate into product molecules.
The product molecules depart from the active site, leaving the enzyme free to catalyse another reaction.
The diagram below illustrates the ‘lock and key’ hypothesis for a reaction in which an enzyme breaks down a substrate molecule into 2 product molecules:
![]()
Effect of Temperature on the rate of reaction:
The effects of temperature on the rate of enzyme-catalysed reactions is shown in the graph below:

At low temperatures, enzymes are inactive and the rate of reaction is very low. Substrate and enzyme molecules have little kinetic energy, hence the frequency of collision is low. In addition, most substrate molecules do not contain sufficient energy to overcome the activation energy required to start a reaction.
As temperature increases, the rate of enzyme activity increases. Enzyme activity doubles with every 10°C rise in temperature. This is because the reactants have higher levels of energy, and the substrate molecules are able to collide with active sites more frequently.
At the optimum temperature, enzyme activity is the highest.
As the temperature increases beyond the optimum temperature, enzyme activity drops sharply. This is because enzymes are made of proteins, which are denatured at high temperatures. The enzyme loses its 3-dimensional structure and active site conformation due to the breaking of the weak bonds that hold the structure together.
At extremely high temperatures, the enzyme is completely denatured and the rate of reaction drops to zero.
Effect of pH on the rate of reaction:
The graph showing the effects of pH on the rate of enzyme-catalysed reactions is shown in the graph below:
![]()
Enzyme activity is the highest at the optimum pH of the enzyme.
As the pH increases or decreases from the optimum, enzyme activity sharply decreases. This is because the hydrogen bonds and ionic bonds that hold the 3-dimensional structure are disrupted. The shape of the active site is changed as the enzyme is denatured.
At extreme pH levels, the enzyme is completely denatured and the rate of reaction drops to zero.
The optimum pH for each enzyme differs. For example, pepsin works best under the acidic conditions in the stomach, while intestinal enzymes work best under alkaline conditions.
 Knowt
Knowt