
Introduction to Organic Chemistry and IUPAC Nomenclature
organic chemistry- chemistry concerning carbon-containing compounds
Why is Carbon Special?
can form strong bonds with multiple atoms of itself in a chain
can bond with many other elements like hydrogen, nitrogen, sulfur, oxygen, and phosphorus
brings diversity in compounds that can be formed
this allows it to be the basis of life
carbon can also form double and triple bonds
carbon is tetravalent- it can remove/gain 4 electrons
functional groups- increase the functionality/reactivity of a molecule
Other Elements
oxygen is divalent- it can gain 2 electrons
nitrogen is trivalent- it can gain 3 electrons
hydrogen is always monovalent- it can lose 1 electron
Types of Structures/Formulas & Other Conventions
lewis structures- show lone pairs as well as number & type of bond present in the molecule
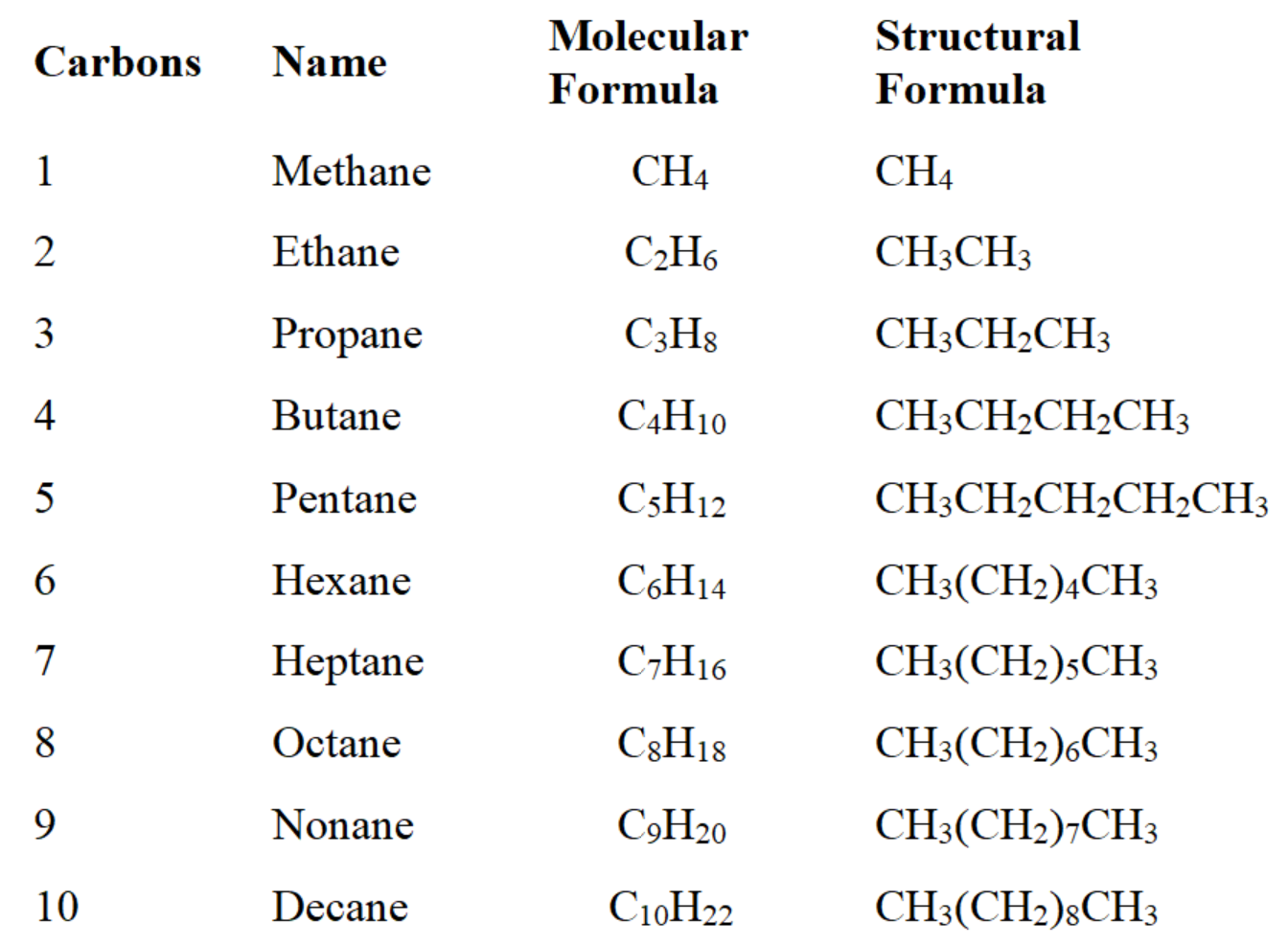
condensed formula- writing the carbons with their hydrogens
example- C20H42O can also be written as CH3(CH2)19OH
**bondlineformula−**shown below
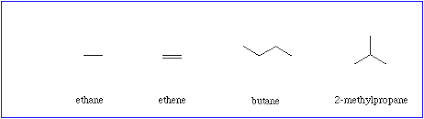
n-(insert molecular formula or name of hydrocarbon) means it’s “normal” & not branched
example- *n-*butane
isomers- molecules with the same molecular formula but different structures
the number of isomers tends to increase as the number of carbons in the compound increases
Hydrocarbons
made of only carbon and hydrogen
not very functionally useful
mostly used for energy
2 types
saturated- maximum amount of hydrogens are in the molecule, all carbons have single bonds
also called alkanes
unsaturated- some carbons have double or triple bonds
IUPAC Nomenclature
parent chain is the longest identifiable carbon chain present in the molecule
if 2 chains have the same length, the parent chain is the one with the most substituents
carbons in the chain are numbered so the substituents get the lowest number possible
if some substituents have the same number no matter numbering from left to right, numbering starts from the end where the next substituent has the lowest number
give the lowest number to the substituent whose letter is first in the alphabet
if more than one of the same type of substituent is present, use the prefixes di- for 2, tri- for 3, tetra- for 4, etc. to indicate the number
substituents are listed in alphabetical order
ignore numerical prefixes in alphabetization (like di-, tri-, tetra-)
don’t ignore positional prefixes like iso-
names and numbers are separated by dashes
multiple numbers are separated by commas
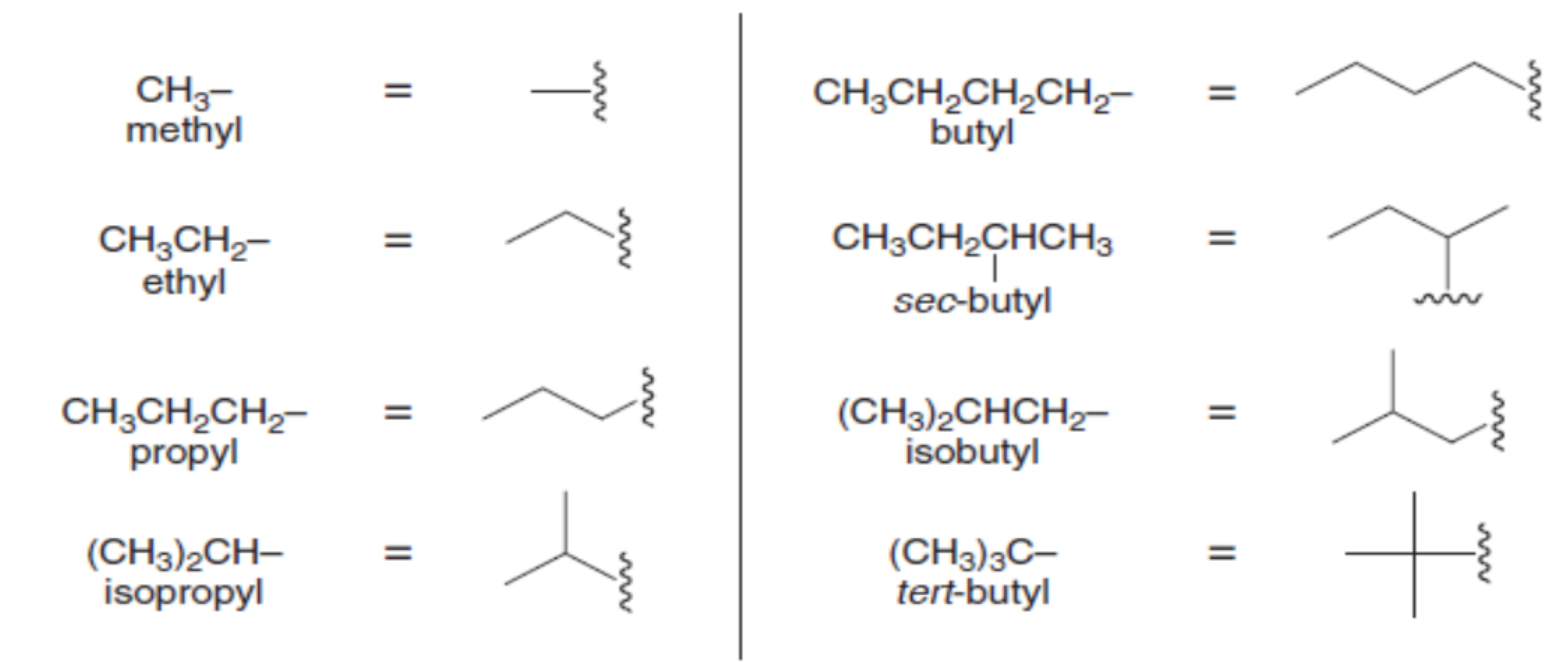
halogens as substituents
F: fluoro-
Cl: chloro-
Br: bromo-
I: iodo-
Example
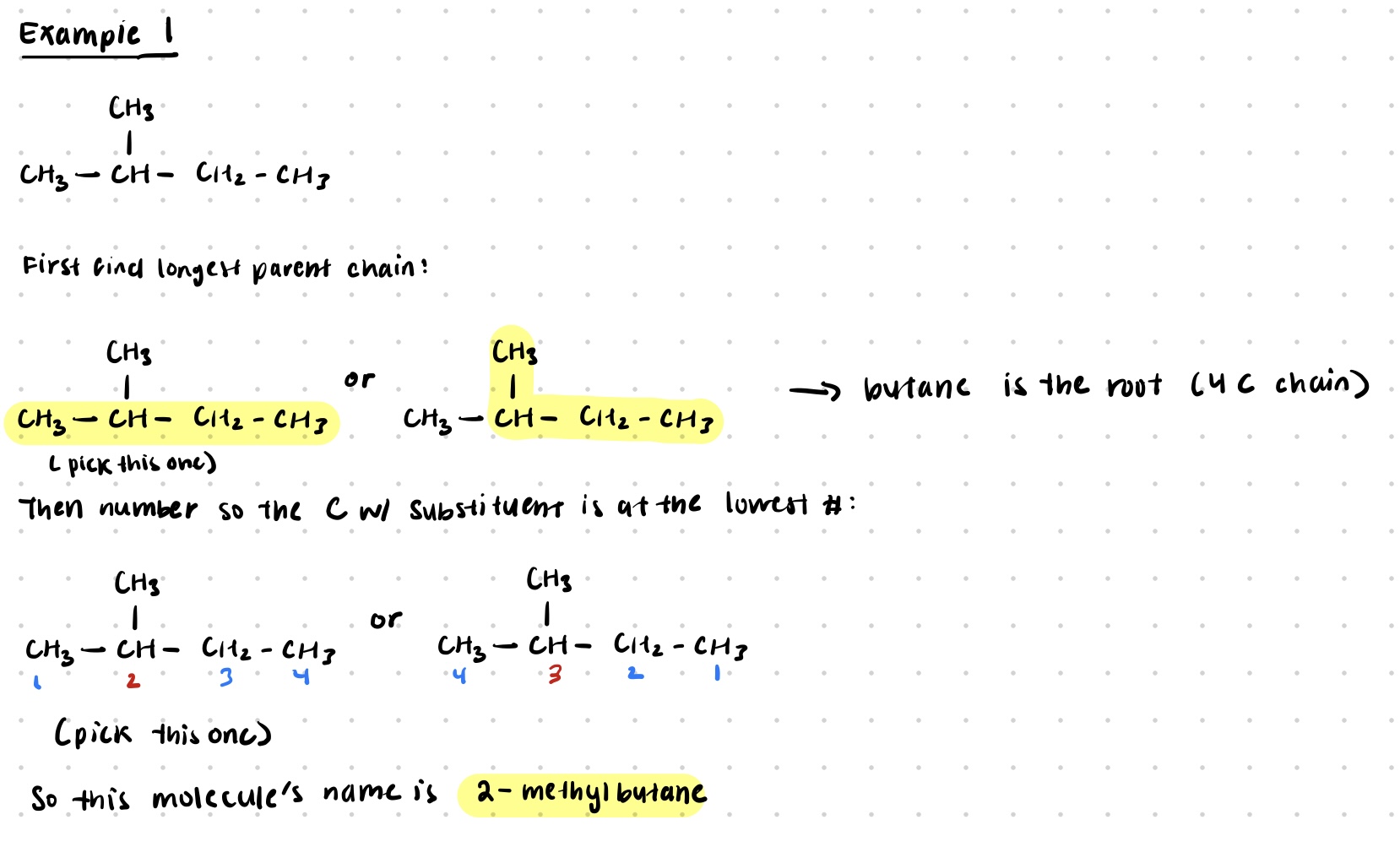
Introduction to Organic Chemistry and IUPAC Nomenclature
organic chemistry- chemistry concerning carbon-containing compounds
Why is Carbon Special?
can form strong bonds with multiple atoms of itself in a chain
can bond with many other elements like hydrogen, nitrogen, sulfur, oxygen, and phosphorus
brings diversity in compounds that can be formed
this allows it to be the basis of life
carbon can also form double and triple bonds
carbon is tetravalent- it can remove/gain 4 electrons
functional groups- increase the functionality/reactivity of a molecule
Other Elements
oxygen is divalent- it can gain 2 electrons
nitrogen is trivalent- it can gain 3 electrons
hydrogen is always monovalent- it can lose 1 electron
Types of Structures/Formulas & Other Conventions
lewis structures- show lone pairs as well as number & type of bond present in the molecule
condensed formula- writing the carbons with their hydrogens
example- C20H42O can also be written as CH3(CH2)19OH
**bondlineformula−**shown below

n-(insert molecular formula or name of hydrocarbon) means it’s “normal” & not branched
example- *n-*butane
isomers- molecules with the same molecular formula but different structures
the number of isomers tends to increase as the number of carbons in the compound increases
Hydrocarbons
made of only carbon and hydrogen
not very functionally useful
mostly used for energy
2 types
saturated- maximum amount of hydrogens are in the molecule, all carbons have single bonds
also called alkanes
unsaturated- some carbons have double or triple bonds
IUPAC Nomenclature
parent chain is the longest identifiable carbon chain present in the molecule
if 2 chains have the same length, the parent chain is the one with the most substituents
carbons in the chain are numbered so the substituents get the lowest number possible
if some substituents have the same number no matter numbering from left to right, numbering starts from the end where the next substituent has the lowest number
give the lowest number to the substituent whose letter is first in the alphabet
if more than one of the same type of substituent is present, use the prefixes di- for 2, tri- for 3, tetra- for 4, etc. to indicate the number
substituents are listed in alphabetical order
ignore numerical prefixes in alphabetization (like di-, tri-, tetra-)
don’t ignore positional prefixes like iso-
names and numbers are separated by dashes
multiple numbers are separated by commas


halogens as substituents
F: fluoro-
Cl: chloro-
Br: bromo-
I: iodo-
Example

 Knowt
Knowt
