
Enthalpy, Entropy, and Heat Review
All the symbols!
q - heat needed
ΔT - Temperature change
ΔH - Enthalpy change
ΔH° - in standard conditions
ΔS - Entropy change
R - Gas constant (8.314 J/Kmol)
K - Dissociation rate
ΔG - Gibbs free energy / available energy
ΔG° - in standard conditions
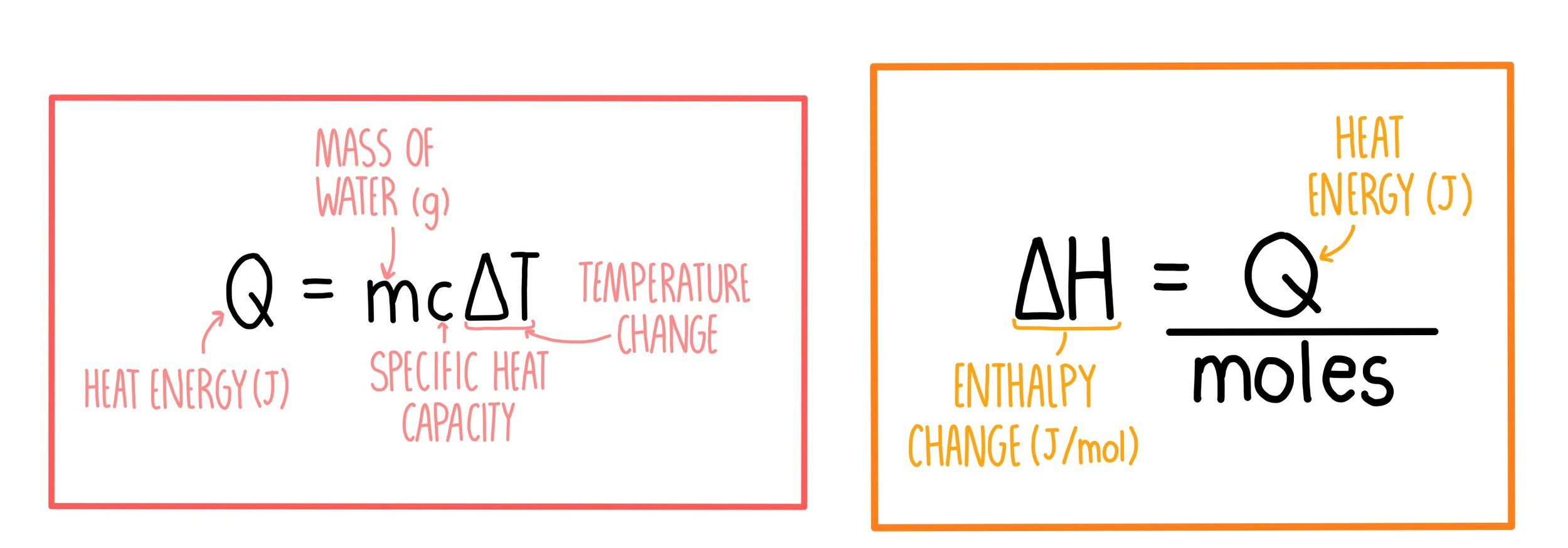
q (Heat)
q = mcΔT
q - heat needed (J)
m - mass of object releasing or absorbing - usually water (4.184 J / gC)
ΔT - temperature change (C)
Molar Heat
ΔH = q / moles
q - heat (J)
ΔH - Enthalpy change (J/mol)
Products - reactants
Coefficient(enthalpy of formation) - coefficient(enthalpy of formation)
You are given the equation and enthalpy of formation for each.
Meaning of ΔH
ΔH > 0 (endothermic / temp of reaction decreases) | ΔH < 0 (exothermic / temp of reaction increases) | |
|---|---|---|
ΔS > 0 (increase in entropy) | ΔG < 0 at high temperaturesΔG > 0 at low temperaturesSpontaneous at high temperatures. | ΔG < 0Spontaneous |
ΔS < 0 (decrease in entropy) | ΔG > 0Nonspontaneous | ΔG < 0 at low temperaturesΔG > 0 at high temperaturesSpontaneous at low temperatures. |
ΔG (Gibbs Free Energy)
ΔG = ΔH - TΔS
ΔG - change in free energy (kJ)
ΔH - change in enthalpy (kJ)
T - absolute temperature (K)
ΔS - change in entropy (kJ/K)
Units can change. Make sure ΔH has same units as ΔS. ΔH is given in kJ/mole + ΔS in J/mole
ΔG° = -RTln(K)
ΔG° applies to standard-state conditions while ΔG is gibbs free energy given certain condition
K - [C][D]/[A][B]
T - temperature (K)
R - constant (8.314 J / mole K)
Meaning of ΔG
Gibb’s free energy.
ΔG < 0 is thermodynamically favored and exothermic.
ΔG > 0 is not thermodynamically favored and endothermic.
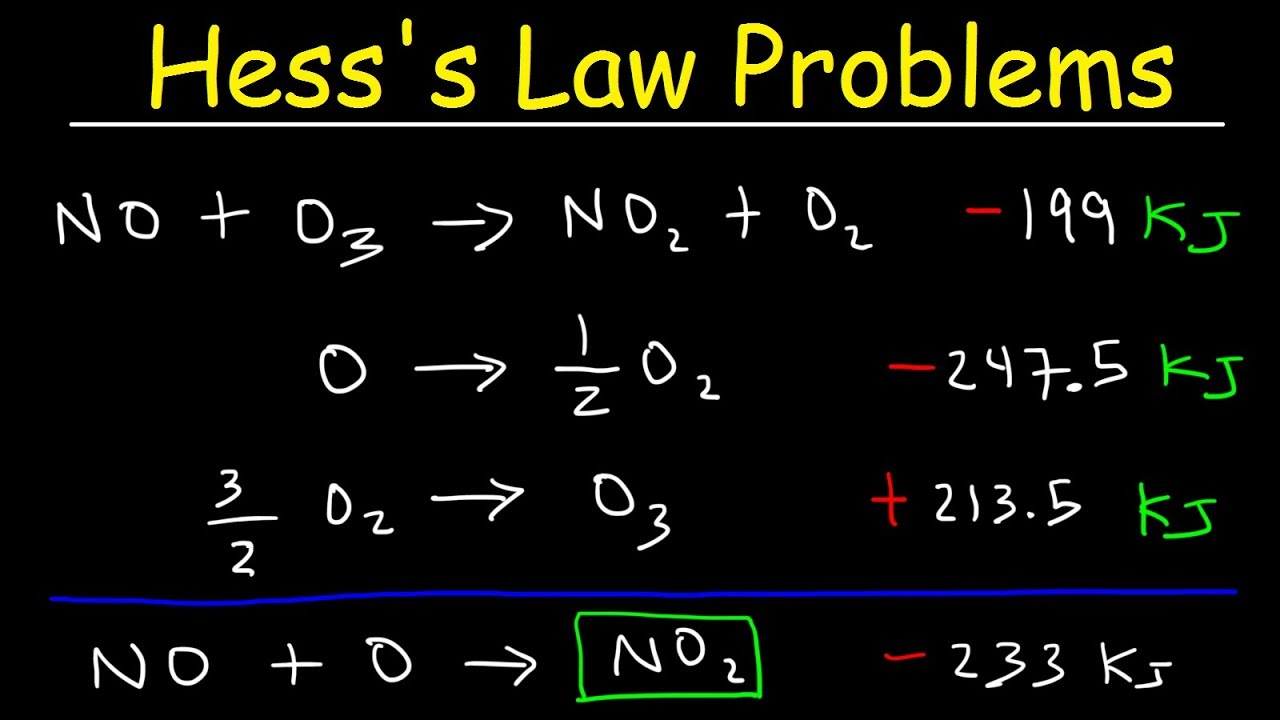
Hess’s Law
Hard to explain. Please click the title in order to watch a short video on Hess’s Law Problems.
ΔS - Change in Entropy
Increases as you go from solid to aqueous to liquid to gas
ΔS = Np(sum of products) - Nr(sum of reactants)
Np = coefficient of products
Nr = coefficient of reactants
Coupled Reactions
Two reactions that share a common intermediate (a product of one reaction is the reactant of another).
Usually combined with Hess’s law to determine free energy change, ΔG, for the coupled reaction.
Heat of reaction / heat of solution
The heat of solution is the amount of heat absorbed or released when a solute dissolves in a solvent, while the heat of reaction is the amount of heat absorbed or released during a chemical reaction. The heat of solution is specific to the dissolution process, while the heat of reaction is specific to the chemical reaction taking place.
Heat of solution
HCl (aq) + NaOH (aq) → NaCl (aq) + H2O (l)
Enthalpy diagram with catalyst
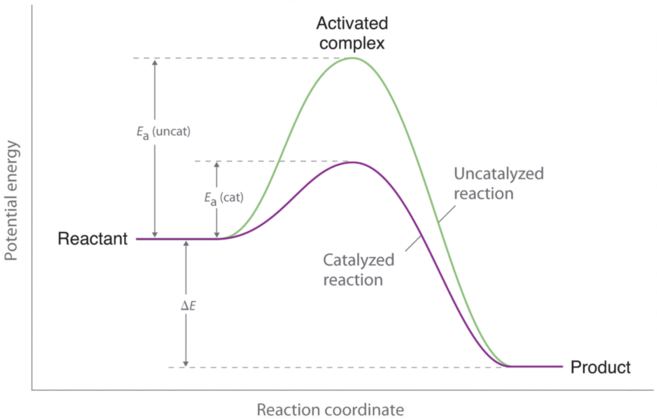
ΔH is the change between starting and ending energy.
Energy of activation is the change in starting energy to the peak.
Starting energy is where the graph starts.
Endothermic reactions have a higher ending energy than starting energy. Exothermic reactions have a lower ending energy than starting energy.
Maxwell Boltzmann
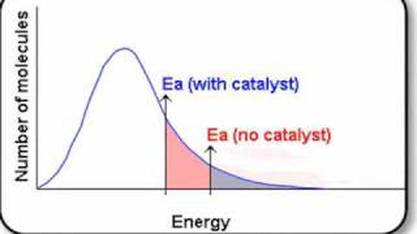
Higher temperatures move the graph peak right and down.
Lattice Enthalpy
Bigger charge triumphs size. Smaller size wins if have same charge.
Drivers
Temperature Change | ΔH | ΔS | Driver |
|---|---|---|---|
↑ | - | - | Enthalpy (G→L→S) |
↓ | + | + | Entropy (S→L→G) |
- | + | Both |
A reaction is favored if enthalpy decreases.
Enthalpy, Entropy, and Heat Review
All the symbols!
q - heat needed
ΔT - Temperature change
ΔH - Enthalpy change
ΔH° - in standard conditions
ΔS - Entropy change
R - Gas constant (8.314 J/Kmol)
K - Dissociation rate
ΔG - Gibbs free energy / available energy
ΔG° - in standard conditions
q (Heat)
q = mcΔT
q - heat needed (J)
m - mass of object releasing or absorbing - usually water (4.184 J / gC)
ΔT - temperature change (C)
Molar Heat
ΔH = q / moles
q - heat (J)
ΔH - Enthalpy change (J/mol)
Products - reactants
Coefficient(enthalpy of formation) - coefficient(enthalpy of formation)
You are given the equation and enthalpy of formation for each.

Meaning of ΔH
ΔH > 0 (endothermic / temp of reaction decreases) | ΔH < 0 (exothermic / temp of reaction increases) | |
|---|---|---|
ΔS > 0 (increase in entropy) | ΔG < 0 at high temperaturesΔG > 0 at low temperaturesSpontaneous at high temperatures. | ΔG < 0Spontaneous |
ΔS < 0 (decrease in entropy) | ΔG > 0Nonspontaneous | ΔG < 0 at low temperaturesΔG > 0 at high temperaturesSpontaneous at low temperatures. |
ΔG (Gibbs Free Energy)
ΔG = ΔH - TΔS
ΔG - change in free energy (kJ)
ΔH - change in enthalpy (kJ)
T - absolute temperature (K)
ΔS - change in entropy (kJ/K)
Units can change. Make sure ΔH has same units as ΔS. ΔH is given in kJ/mole + ΔS in J/mole
ΔG° = -RTln(K)
ΔG° applies to standard-state conditions while ΔG is gibbs free energy given certain condition
K - [C][D]/[A][B]
T - temperature (K)
R - constant (8.314 J / mole K)
Meaning of ΔG
Gibb’s free energy.
ΔG < 0 is thermodynamically favored and exothermic.
ΔG > 0 is not thermodynamically favored and endothermic.
Hess’s Law
Hard to explain. Please click the title in order to watch a short video on Hess’s Law Problems.

ΔS - Change in Entropy
Increases as you go from solid to aqueous to liquid to gas
ΔS = Np(sum of products) - Nr(sum of reactants)
Np = coefficient of products
Nr = coefficient of reactants
Coupled Reactions
Two reactions that share a common intermediate (a product of one reaction is the reactant of another).
Usually combined with Hess’s law to determine free energy change, ΔG, for the coupled reaction.
Heat of reaction / heat of solution
The heat of solution is the amount of heat absorbed or released when a solute dissolves in a solvent, while the heat of reaction is the amount of heat absorbed or released during a chemical reaction. The heat of solution is specific to the dissolution process, while the heat of reaction is specific to the chemical reaction taking place.
Heat of solution
HCl (aq) + NaOH (aq) → NaCl (aq) + H2O (l)
Enthalpy diagram with catalyst

ΔH is the change between starting and ending energy.
Energy of activation is the change in starting energy to the peak.
Starting energy is where the graph starts.
Endothermic reactions have a higher ending energy than starting energy. Exothermic reactions have a lower ending energy than starting energy.
Maxwell Boltzmann

Higher temperatures move the graph peak right and down.
Lattice Enthalpy
Bigger charge triumphs size. Smaller size wins if have same charge.
Drivers
Temperature Change | ΔH | ΔS | Driver |
|---|---|---|---|
↑ | - | - | Enthalpy (G→L→S) |
↓ | + | + | Entropy (S→L→G) |
- | + | Both |
A reaction is favored if enthalpy decreases.
 Knowt
Knowt
