Honors Biology Notes Grade 10 Highschool Unit 1:
U 1: Biochemistry
Characteristics of Life
Highly Organized
Composed of Cells
Reproduce
Based on genetic code like DNA and RNA
Growth and development
Obtain and use energy
Respond to stimuli
Maintain Homeostasis
Have a limited lifespan
Chemistry Review
Define element: a substance that cannot be broken down into any other substance
What is an atom? the basic unit of a chemical element
Most common elements found in living organisms: CHONPS
Carbon Hydrogen Oxygen
Nitrogen
Phosphorus
Sulfur
What are the 3 types of bonds that form between atoms?
Covalent:
Atoms share valence electrons. There can be more than one covalent bond per molecule (single bond, double bond, triple bond) VERY STRONG BOND, but not as strong as ionic.
Ionic:
One atom “accepts” or “donates” one or more valence electrons to another atom. Ion–charged particle STRONGEST BOND
Hydrogen:
interaction involving a hydrogen atom located between a pair of other atoms having a high affinity for electrons
Characteristics of water definitions:
Cohesion:
Water bonding to water
Adhesion:
Water bonding to something else
Universal solvent:
Water’s polarity allows it to be attracted to other polar or ionic compounds. Since water is small and polar, it easily surrounds other molecules and the attraction between the water and the charged atoms in the compound will split the compound apart (dissolve it).
Biochemistry
What is the easiest way to identify a molecule as “organic”? Organic means “contains carbon” 90% of all know compounds are organic
Define functional group: a group of atoms in a chemical that defines its characteristics
Define the following root words:
Macro: “big,large"
Mono: “one”
Poly: “many”
Monomer | Polymer | |
|---|---|---|
Define | building block | chain of monomers |
Diagram | 0 | 0-0-0-0-0-0 |
CARBOHYDRATES
Made of: C, H, O (ratio of 1:2:1)
Monomer (Subunit): monosaccharide
Function: to provide energy for the body 3 sizes:
Monosaccharide
Smallest Carbohydrate
Glucose, Fructose, and Galactose are 3 monosaccharides
Disaccharide
Two monosaccharides bonded together
Sucrose Lactose Maltose are 3 important disaccharides: (these all make up sugary foods)
Polysaccharide
Many monosaccharides bonded together also called “complex carbohydrates”
Chain of many highly branched glucose molecules
Starch Glycogen Cellulose are 3 polysaccharides
“Saccharide” = “sugar” “mono” = “one” “di” = “two” “poly” = “many”
How are the functions of sugars and starches different? Sugar is already in the form needed by the body for metabolism, so it doesn't need to be digested – it only needs to be absorbed. On the other hand, starch does require digestion, as it must be broken down into sugar in order to be absorbed.
Lipids
Elements: C, H, O
Monomers (subunits): Fatty acid and glycerol
Ratio: 1:2 for C: H, and much less oxygen
-Example: C50: H100: O6 (about twice as much H as C) (Does NOT have 1:2:1 C: H:O ratio like carbohydrates)
Hydrophobic: means that they do not dissolve in water
5 Types of Lipids:
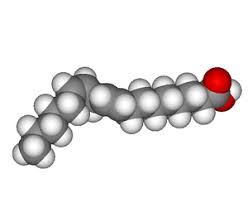
Unsaturated Fats (Oils)
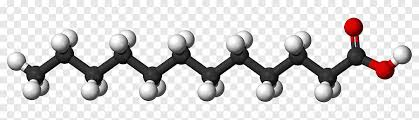
Saturated Fats
Waxes
Steroids
Phospholipids
Type of Lipid | Function/Examples | Diagram |
|---|---|---|
Unsaturated Fats | - Double Bonds (not closely packed) - Healthy - Bent structure at the end |
|
Saturated Fats | - Single Bonds (close packing)- Unhealthy - Straight structure |
|
Waxes | - protective coating- waterproof coating on leaves, fruits, and feathers−Protectivecoatingforearcanal - Beeswax | |
Steroids/Sterols | - Regulate processes in the body- Hormones: estrogen, testosterone}} | |
Phospholipids | ^^- form the cell membranes of organisms |
Proteins:
Made of: C, H, O, N
Subunit: Amino acids
Primary functions of proteins: Help the growth and maintenance of cells
Monomer: Amino Acid (also known as a peptide)
Polymer: Protein (also known as a polypeptide or amino acid chain)
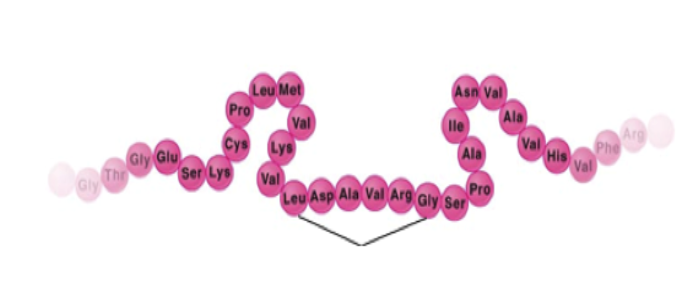
The picture on the right is a primary structure of a protein. It is made up of a sequence of amino acids joined by a peptide bond. A bond between 2 amino acids is called a “peptide bond” |
|
|---|
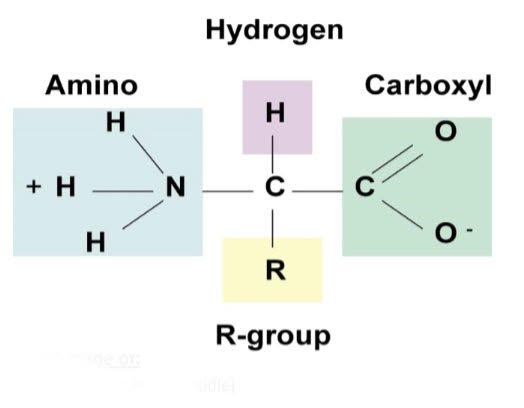
The picture on the right is the amino acid structure. Amino acids are made of: - A central carbon (one carbon in the middle)- 4 groups attached to the carbon: - Amino group (NH2 or NH3+) - Carboxyl group (COOH or COO-) - Hydrogen group (H) - Random group (Also called a “side group” or “R-group”) |
|
|---|
The table below are some protein examples:
Types of Proteins: | Function/Examples | Diagram |
|---|---|---|
Keratin | Gives Strength to:****- Skin- Hair/Fur- Nails - Hooves- Horns- Teeth | |
Muscles | Tissue that allows movement of and within the body: - Skeletal muscle (attached to bones) - Cardiac muscle (makes up the heart) - Smooth muscle (makes up stomach, diaphragm) | |
Hemoglobin | - In blood cells - Helps transport oxygen to tissues in body | |
Antibodies | - Help defend the body against diseases and infections |
The effect of temperature on proteins:
As temperature rises, this affects the stability of the hydrogen bonds that are holding the secondary and tertiary structures in place.
The result of an increase in temperature could be denaturation or unraveling of the protein to the primary structure. If this occurs, the protein loses its ability to properly function within the body.
The table below displays the different protein structures
Protein Structure | Description | Diagram/Picture |
|---|---|---|
Primary | Primary (1____) (the first step)*- The amino acids are put in order (like putting beads on a necklace) |
|
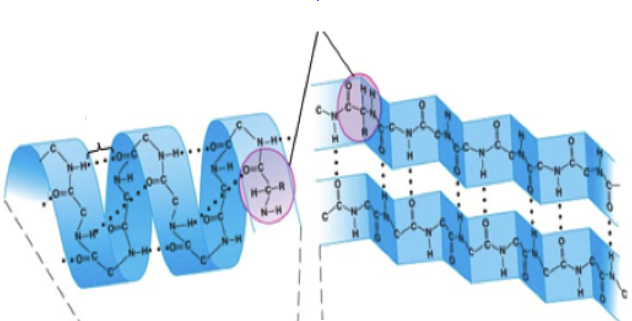
Secondary | Secondary (2____) (the second step)*- The primary structure folds or coils up. - Some parts coil (called alpha helices)- Other parts fold (Beta-pleated sheets) |
|
Tertiary | Tertiary (3____) (the third step)*- The secondary structure folds up on itself (to make a big ball) |
|
Quaternary | __Quaternary (4) (the fourth step)*__Two or more tertiary structures bonded together. |
|
Enzyme:
Function: speed up the rate of reactions
Enzyme: The specific protein responsible for breaking down/building a reactant in a living thing
Substrate(s): The reactant in a chemical reaction performed by an enzyme
Active Site: The physical place on the enzyme that the substrate molecule fits into
Enzyme-Substrate Complex: The single structure formed when an enzyme is completely bonded to the substrate, before the substrate(s) change into the product(s)
Product(s): The resulting molecules that are formed in a chemical reaction (released from the enzyme)
How do enzymes generally work?
Enzymes use the processes of dehydration synthesis and hydrolysis to preform their reactions
]]Dehydration (building molecules) ]] - synthesis enzymes |
|
|---|---|
Hydrolysis (digesting molecules) - digesting enzymes |
|
Note: In the pictures above, an arrow with the word “enzyme” above the blue arrow indicates that the enzyme is the molecule that turns the reactants into the products.
Honors Biology Notes Grade 10 Highschool Unit 1:
U 1: Biochemistry
Characteristics of Life
Highly Organized
Composed of Cells
Reproduce
Based on genetic code like DNA and RNA
Growth and development
Obtain and use energy
Respond to stimuli
Maintain Homeostasis
Have a limited lifespan
Chemistry Review
Define element: a substance that cannot be broken down into any other substance
What is an atom? the basic unit of a chemical element
Most common elements found in living organisms: CHONPS
Carbon Hydrogen Oxygen
Nitrogen
Phosphorus
Sulfur
What are the 3 types of bonds that form between atoms?
Covalent:
Atoms share valence electrons. There can be more than one covalent bond per molecule (single bond, double bond, triple bond) VERY STRONG BOND, but not as strong as ionic.
Ionic:
One atom “accepts” or “donates” one or more valence electrons to another atom. Ion–charged particle STRONGEST BOND
Hydrogen:
interaction involving a hydrogen atom located between a pair of other atoms having a high affinity for electrons
Characteristics of water definitions:
Cohesion:
Water bonding to water
Adhesion:
Water bonding to something else
Universal solvent:
Water’s polarity allows it to be attracted to other polar or ionic compounds. Since water is small and polar, it easily surrounds other molecules and the attraction between the water and the charged atoms in the compound will split the compound apart (dissolve it).
Biochemistry
What is the easiest way to identify a molecule as “organic”? Organic means “contains carbon” 90% of all know compounds are organic
Define functional group: a group of atoms in a chemical that defines its characteristics
Define the following root words:
Macro: “big,large"
Mono: “one”
Poly: “many”
Monomer | Polymer | |
|---|---|---|
Define | building block | chain of monomers |
Diagram | 0 | 0-0-0-0-0-0 |
CARBOHYDRATES
Made of: C, H, O (ratio of 1:2:1)
Monomer (Subunit): monosaccharide
Function: to provide energy for the body 3 sizes:
Monosaccharide
Smallest Carbohydrate
Glucose, Fructose, and Galactose are 3 monosaccharides
Disaccharide
Two monosaccharides bonded together
Sucrose Lactose Maltose are 3 important disaccharides: (these all make up sugary foods)
Polysaccharide
Many monosaccharides bonded together also called “complex carbohydrates”
Chain of many highly branched glucose molecules
Starch Glycogen Cellulose are 3 polysaccharides
“Saccharide” = “sugar” “mono” = “one” “di” = “two” “poly” = “many”
How are the functions of sugars and starches different? Sugar is already in the form needed by the body for metabolism, so it doesn't need to be digested – it only needs to be absorbed. On the other hand, starch does require digestion, as it must be broken down into sugar in order to be absorbed.
Lipids
Elements: C, H, O
Monomers (subunits): Fatty acid and glycerol
Ratio: 1:2 for C: H, and much less oxygen
-Example: C50: H100: O6 (about twice as much H as C) (Does NOT have 1:2:1 C: H:O ratio like carbohydrates)
Hydrophobic: means that they do not dissolve in water
5 Types of Lipids:
Unsaturated Fats (Oils)
Saturated Fats
Waxes
Steroids
Phospholipids
Type of Lipid | Function/Examples | Diagram |
|---|---|---|
Unsaturated Fats | - Double Bonds (not closely packed) - Healthy - Bent structure at the end |
|
Saturated Fats | - Single Bonds (close packing)- Unhealthy - Straight structure |
|
Waxes | - protective coating- waterproof coating on leaves, fruits, and feathers−Protectivecoatingforearcanal - Beeswax | |
Steroids/Sterols | - Regulate processes in the body- Hormones: estrogen, testosterone}} | |
Phospholipids | ^^- form the cell membranes of organisms |
Proteins:
Made of: C, H, O, N
Subunit: Amino acids
Primary functions of proteins: Help the growth and maintenance of cells
Monomer: Amino Acid (also known as a peptide)
Polymer: Protein (also known as a polypeptide or amino acid chain)
The picture on the right is a primary structure of a protein. It is made up of a sequence of amino acids joined by a peptide bond. A bond between 2 amino acids is called a “peptide bond” |
|
|---|
The picture on the right is the amino acid structure. Amino acids are made of: - A central carbon (one carbon in the middle)- 4 groups attached to the carbon: - Amino group (NH2 or NH3+) - Carboxyl group (COOH or COO-) - Hydrogen group (H) - Random group (Also called a “side group” or “R-group”) |
|
|---|
The table below are some protein examples:
Types of Proteins: | Function/Examples | Diagram |
|---|---|---|
Keratin | Gives Strength to:****- Skin- Hair/Fur- Nails - Hooves- Horns- Teeth | |
Muscles | Tissue that allows movement of and within the body: - Skeletal muscle (attached to bones) - Cardiac muscle (makes up the heart) - Smooth muscle (makes up stomach, diaphragm) | |
Hemoglobin | - In blood cells - Helps transport oxygen to tissues in body | |
Antibodies | - Help defend the body against diseases and infections |
The effect of temperature on proteins:
As temperature rises, this affects the stability of the hydrogen bonds that are holding the secondary and tertiary structures in place.
The result of an increase in temperature could be denaturation or unraveling of the protein to the primary structure. If this occurs, the protein loses its ability to properly function within the body.
The table below displays the different protein structures
Protein Structure | Description | Diagram/Picture |
|---|---|---|
Primary | Primary (1____) (the first step)*- The amino acids are put in order (like putting beads on a necklace) |
|
Secondary | Secondary (2____) (the second step)*- The primary structure folds or coils up. - Some parts coil (called alpha helices)- Other parts fold (Beta-pleated sheets) |
|
Tertiary | Tertiary (3____) (the third step)*- The secondary structure folds up on itself (to make a big ball) |
|
Quaternary | __Quaternary (4) (the fourth step)*__Two or more tertiary structures bonded together. |
|
Enzyme:
Function: speed up the rate of reactions
Enzyme: The specific protein responsible for breaking down/building a reactant in a living thing
Substrate(s): The reactant in a chemical reaction performed by an enzyme
Active Site: The physical place on the enzyme that the substrate molecule fits into
Enzyme-Substrate Complex: The single structure formed when an enzyme is completely bonded to the substrate, before the substrate(s) change into the product(s)
Product(s): The resulting molecules that are formed in a chemical reaction (released from the enzyme)
How do enzymes generally work?
Enzymes use the processes of dehydration synthesis and hydrolysis to preform their reactions
]]Dehydration (building molecules) ]] - synthesis enzymes |
|
|---|---|
Hydrolysis (digesting molecules) - digesting enzymes |
|
Note: In the pictures above, an arrow with the word “enzyme” above the blue arrow indicates that the enzyme is the molecule that turns the reactants into the products.
 Knowt
Knowt