
Chapter 15- Electrolysis
Electrolysis: the process of splitting ionic compounds (molten or aqueous) into their constituent elements using electricity.
Electrodes: a conductor through which electricity enters or leaves during electrolysis. The positive electrode is the anode and the negative electrode is the cathode. Usually, inert electrodes are used so they do not react.
Electrolyte: a liquid or which contains ions and can be decomposed by electrolysis
Electrolytic cell: the site at which electrolysis takes place. It has the electrodes, battery and electrolyte attached or in contact with it.
Discharge: the process of gaining or losing (reduction and oxidation respectively) at the electrodes.
Selective discharge: the preferably discharged anion or cation when more than one of each ion is present in the electrolyte.
Simple/electric cell: a device that converts chemical energy to electrical energy.
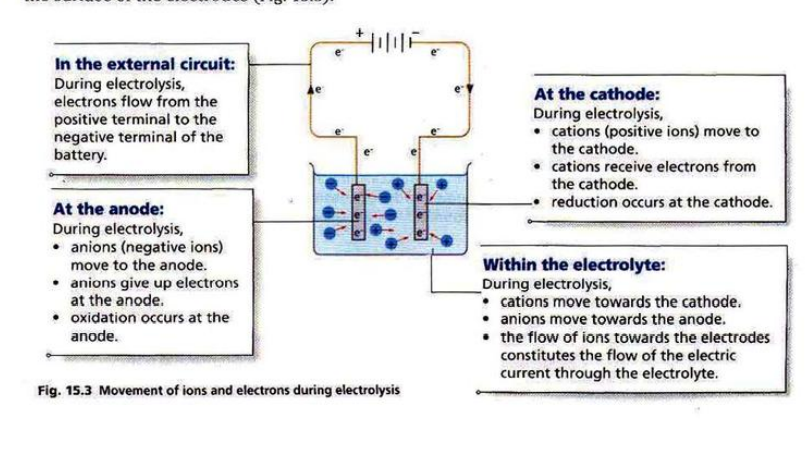
In molten electrolytes, only one cation and anion are present which is discharged at the electrodes.
However, in aqueous solutions, the H+ and OH- ions of water are also present. Therefore, selective discharge occurs based on the position of the elements in the reactivity series.


APPLICATIONS OF ELECTROLYSIS:
Electroplating:
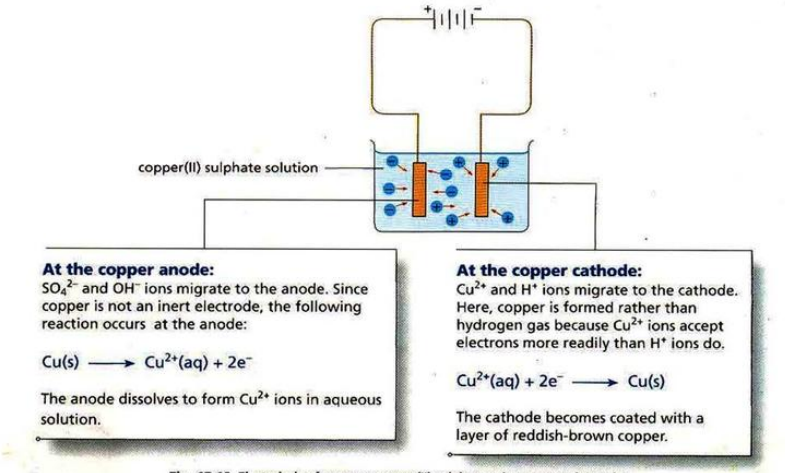
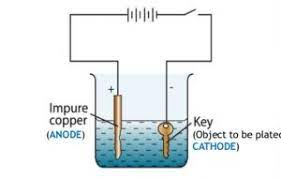 Purification of copper:
Purification of copper:
Extraction of Aluminium:

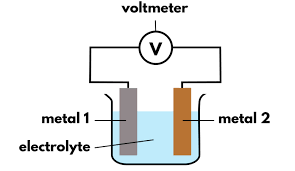
SIMPLE CELL
The voltage in a simple cell depends on the difference between the positions of the two metals in the reactivity series. The greater the difference in the series, the greater the voltage. For example, a simple cell of zinc and copper will produce a greater voltage than a cell with zinc and magnesium.
Chapter 15- Electrolysis
Electrolysis: the process of splitting ionic compounds (molten or aqueous) into their constituent elements using electricity.
Electrodes: a conductor through which electricity enters or leaves during electrolysis. The positive electrode is the anode and the negative electrode is the cathode. Usually, inert electrodes are used so they do not react.
Electrolyte: a liquid or which contains ions and can be decomposed by electrolysis
Electrolytic cell: the site at which electrolysis takes place. It has the electrodes, battery and electrolyte attached or in contact with it.
Discharge: the process of gaining or losing (reduction and oxidation respectively) at the electrodes.
Selective discharge: the preferably discharged anion or cation when more than one of each ion is present in the electrolyte.
Simple/electric cell: a device that converts chemical energy to electrical energy.

In molten electrolytes, only one cation and anion are present which is discharged at the electrodes.
However, in aqueous solutions, the H+ and OH- ions of water are also present. Therefore, selective discharge occurs based on the position of the elements in the reactivity series.


APPLICATIONS OF ELECTROLYSIS:
Electroplating:
 Purification of copper:
Purification of copper:

Extraction of Aluminium:

SIMPLE CELL
The voltage in a simple cell depends on the difference between the positions of the two metals in the reactivity series. The greater the difference in the series, the greater the voltage. For example, a simple cell of zinc and copper will produce a greater voltage than a cell with zinc and magnesium.

 Knowt
Knowt
