
PY 131 Chapter 17: Phase Changes
Phases
Most substances can exist in different states e.g. as a solid or liquid, depending upon the temperature and pressure.
A figure showing the state as a function of T and P is called a phase diagram.

Some substances, e.g. hydrogen, have many more phases so their phase diagrams can get a bit complicated.

Phase Changes (Transitions)
A substance can change its phase, from e.g. solid to liquid or solid to gas.
This can be accomplished by heating or cooling it (changing its temperature) or squeezing it (changes in pressure).
For some substances (e.g. mixtures) phase changes can occur by changing some other property (e.g. concentration)
Melting/Freezing
Melting is the phase change a substance undergoes when it transitions from a solid to a liquid.
Freezing is the opposite.
During melting/freezing the temperature of the substance remains constant until all the substance has changed phase.
Melting/Freezing temperatures or pure substances can be altered by impurities e.g. salt in water.
Melting is usually accomplished by adding heat.
When something freezes the heat must be removed
The heat added to 1 kg of a substance to cause melting is called the latent heat of fusion.
The energy is used to break interatomic/intermolecular bonds.
The latent heat of fusion for water is 335,000 J/kg
When a substance freezes the latent heat of fusion is absorbed by the environment.
For a few substances such as water, melting/freezing can also be accomplished by applying/removing pressure.
Water expands upon freezing which is not common.
Applying pressure to ice increases its density.
When the density of the liquid phase is reached, the ice melts.
At 500 atmospheres the melting point of water is -4 °C.
When the pressure is removed the liquid refreezes into a solid.
Melting due to applied pressure and refreezing when it is removed is called regulation.
Regelation is different from surface melting (which makes ice slippery).
Regelation can occur to the ice under a glacier but making snowballs does not involve regulation.
Boiling/Condensing
The phase change between a liquid and a gas is called boiling.
Condensing is the change from a gas to a liquid.
The boiling point of most liquids can be changed with pressure and with the addition of impurities.
The energy required to change 1 kg of a liquid to a gas is called the latent heat of vaporization.
The latent heat of vaporization for water is a 2,260,000 J/kg
The latent heat of vaporization for a substance is always much larger than the latent heat of fusion.
When a substance condenses the latent heat is released into the environment.
The temperature versus heating graph for water shows the two phases’ changes.
It takes more energy to boil 1 kg of water than it does to melt 1 kg of ice and then raise its temperature to 100 °C.

EXAMPLE 1
When rain forms in clouds, the surrounding air is
A. cooled.
B. warmed.
C. insulated.
D. thermally conducting.
The condensation of water droplets releases the energy of vaporization
Evaporation
Evaporation is a different process than boiling.
During evaporation fast-moving atoms/molecules at a liquid surface escape to a gas phase.
The loss of above-average energy atoms/molecules lowers the average of the remainder.
Evaporation thus cools the remaining liquid.
If the remaining liquid is in thermal contact with another object at higher temperature, heat will be withdrawn from the hotter object.
Dew points
As the temperature of a mixture of two (or more) gases is reduced, one of the gases may form droplets of its liquid phase.
For a given mixture of gases, the temperature at which this occurs is called the dew point.
If the dew point temperature is below the freezing point it is called the frost point.
A similar effect can occur in mixtures of liquids when one component of the liquid will freeze into its solid phase or the liquid phase separates (cease to mix).
The temperature at which this occurs is called the cloud point.
Examples include cooled oils.
Other Phase Changes
The phase changes from solid to liquid, or liquid to gas (and vice versa) are not the only possibility.
Other phase changes include:
from solid to gas (sublimation/deposition)
from one solid phase to another solid phase,
from a neutral gas to a plasma (ionization/recombination)
from magnetized to non-magnetized
between different molecular structures e.g. molecular hydrogen to atomic hydrogen, O2 to O3
quantum phase transitions where one phase exhibits quantum behavior e.g. superconductivity, superfluidity, quantum condensation
Sublimation/Deposition
The phase change from solid to gas is called sublimation.
Deposition is the change from gas to solid.
Whether a substance sublimates or melts depends on the (partial) pressure of the gas above the solid.
The energy required to sublimate 1 kg of a substance is called the heat of sublimation.
In freeze drying, an object containing water is first frozen in a chamber then the pressure in the container is reduced and the ice sublimates.
Ionization/Recombination
Charged atoms/molecules are called ions. Electrons that are not bound to an atom/molecule are called free electrons.
A mixture of free electrons and ions is plasma.
The removal of electrons from gaseous atoms/molecules is called ionization and is a phase change.
Ionization is different from the dissociation of e.g. solid NaCl in water.
The energy required to remove an electron from a single atom/molecule is called the ionization energy.
Ionization can be accomplished by adding heat but there are other alternatives.
e.g. shining light, and collisions with energetic particles.
Like other phase changes, the temperature remains constant during the phase change.
The ionization temperature of hydrogen gas is ~10,000 K.
When electrons are captured by ions the process is called recombination.
Recombination is the opposite of ionization.
Usually, the energy released by recombination is emitted in the form of light.
Special Points in a Phase Diagram
There are two types of special points in a phase diagram: triple points and critical points.
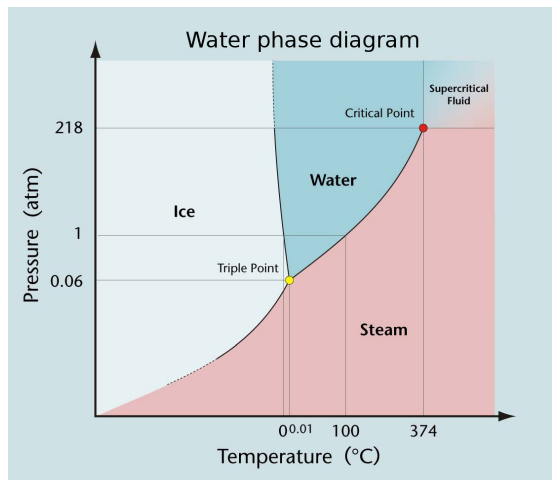
Triple Points
For every substance that can exist in three phases (e.g. as a solid, liquid and a gas) there is a unique temperature and pressure at which all three phases can coexist.
This combination of temperature and pressure is called the substance’s triple point.
You can have triple points between any three phases of the substance e.g. two solid phases and the gas.
Helium has a triple point between two liquid phases and the gas.
The triple point of pure water used to be the second fixed point on the Kelvin temperature scale.
The temperature of the triple point of water was defined to be 273.16 K
Critical Points
For every substance that can exist as a liquid and a gas, there is a unique temperature and pressure at which the distinction between liquid and gas disappears.
This combination of temperature and pressure is called the substance’s critical point.
You can also have critical points between any other two phases of the substance e.g. two solid phases or two liquid phases
For temperatures above the critical point, gas cannot be liquefied by pressure alone.
A substance with a temperature and pressure larger than the liquid-vapor critical point is sometimes called a supercritical fluid.
The critical point of pure water is 373.946 °C and 22,060 kPa.
Supercritical water can be found around ‘black smokers’, geothermally heated water issuing from vents on the sea floor.
Superheating/Supercooling
Phase changes don’t occur uniformly in a substance, they are usually patchy e.g. when a liquid boils it forms bubbles.
Bubbles in a boiling liquid often form around imperfections of some kind which form nucleation sites.
Bubbles start small and then grow (they expand and the amount of gas in the bubble increases due to evaporation from the bubble surface)
The gas pressure must overcome the liquid pressure and also overcome the surface tension of the liquid.
Because a small bubble has a large surface-to-volume ratio, it takes a larger gas pressure to make a small bubble than a big bubble.
If small bubbles cannot form, the liquid does not undergo the phase transition and can be heated to a temperature higher than the boiling point.
Such a liquid is called superheated.
Superheated liquid hydrogen was once used in high-energy particle detectors known as ‘bubble chambers’.
Particles traveling through the superheated liquid create nucleation sites which then form bubbles.
Photographs of the bubbles reveals the path of the particle.
A similar phenomenon can occur when a gas tries to condense into a liquid or when liquids try to freeze into solids.
Without nucleation sites the gas can be cooled below the boiling temperature or the liquid below its freezing temperature.
Such a gas / liquid is called supercooled.
Pure water can be supercooled to −48.3 °C.
Supercooled carbon dioxide gas was once used in high energy particle detectors known as ‘cloud chambers’.
PY 131 Chapter 17: Phase Changes
Phases
Most substances can exist in different states e.g. as a solid or liquid, depending upon the temperature and pressure.
A figure showing the state as a function of T and P is called a phase diagram.

Some substances, e.g. hydrogen, have many more phases so their phase diagrams can get a bit complicated.

Phase Changes (Transitions)
A substance can change its phase, from e.g. solid to liquid or solid to gas.
This can be accomplished by heating or cooling it (changing its temperature) or squeezing it (changes in pressure).
For some substances (e.g. mixtures) phase changes can occur by changing some other property (e.g. concentration)
Melting/Freezing
Melting is the phase change a substance undergoes when it transitions from a solid to a liquid.
Freezing is the opposite.
During melting/freezing the temperature of the substance remains constant until all the substance has changed phase.
Melting/Freezing temperatures or pure substances can be altered by impurities e.g. salt in water.
Melting is usually accomplished by adding heat.
When something freezes the heat must be removed
The heat added to 1 kg of a substance to cause melting is called the latent heat of fusion.
The energy is used to break interatomic/intermolecular bonds.
The latent heat of fusion for water is 335,000 J/kg
When a substance freezes the latent heat of fusion is absorbed by the environment.
For a few substances such as water, melting/freezing can also be accomplished by applying/removing pressure.
Water expands upon freezing which is not common.
Applying pressure to ice increases its density.
When the density of the liquid phase is reached, the ice melts.
At 500 atmospheres the melting point of water is -4 °C.
When the pressure is removed the liquid refreezes into a solid.
Melting due to applied pressure and refreezing when it is removed is called regulation.
Regelation is different from surface melting (which makes ice slippery).
Regelation can occur to the ice under a glacier but making snowballs does not involve regulation.
Boiling/Condensing
The phase change between a liquid and a gas is called boiling.
Condensing is the change from a gas to a liquid.
The boiling point of most liquids can be changed with pressure and with the addition of impurities.
The energy required to change 1 kg of a liquid to a gas is called the latent heat of vaporization.
The latent heat of vaporization for water is a 2,260,000 J/kg
The latent heat of vaporization for a substance is always much larger than the latent heat of fusion.
When a substance condenses the latent heat is released into the environment.
The temperature versus heating graph for water shows the two phases’ changes.
It takes more energy to boil 1 kg of water than it does to melt 1 kg of ice and then raise its temperature to 100 °C.

EXAMPLE 1
When rain forms in clouds, the surrounding air is
A. cooled.
B. warmed.
C. insulated.
D. thermally conducting.
The condensation of water droplets releases the energy of vaporization
Evaporation
Evaporation is a different process than boiling.
During evaporation fast-moving atoms/molecules at a liquid surface escape to a gas phase.
The loss of above-average energy atoms/molecules lowers the average of the remainder.
Evaporation thus cools the remaining liquid.
If the remaining liquid is in thermal contact with another object at higher temperature, heat will be withdrawn from the hotter object.
Dew points
As the temperature of a mixture of two (or more) gases is reduced, one of the gases may form droplets of its liquid phase.
For a given mixture of gases, the temperature at which this occurs is called the dew point.
If the dew point temperature is below the freezing point it is called the frost point.
A similar effect can occur in mixtures of liquids when one component of the liquid will freeze into its solid phase or the liquid phase separates (cease to mix).
The temperature at which this occurs is called the cloud point.
Examples include cooled oils.
Other Phase Changes
The phase changes from solid to liquid, or liquid to gas (and vice versa) are not the only possibility.
Other phase changes include:
from solid to gas (sublimation/deposition)
from one solid phase to another solid phase,
from a neutral gas to a plasma (ionization/recombination)
from magnetized to non-magnetized
between different molecular structures e.g. molecular hydrogen to atomic hydrogen, O2 to O3
quantum phase transitions where one phase exhibits quantum behavior e.g. superconductivity, superfluidity, quantum condensation
Sublimation/Deposition
The phase change from solid to gas is called sublimation.
Deposition is the change from gas to solid.
Whether a substance sublimates or melts depends on the (partial) pressure of the gas above the solid.
The energy required to sublimate 1 kg of a substance is called the heat of sublimation.
In freeze drying, an object containing water is first frozen in a chamber then the pressure in the container is reduced and the ice sublimates.
Ionization/Recombination
Charged atoms/molecules are called ions. Electrons that are not bound to an atom/molecule are called free electrons.
A mixture of free electrons and ions is plasma.
The removal of electrons from gaseous atoms/molecules is called ionization and is a phase change.
Ionization is different from the dissociation of e.g. solid NaCl in water.
The energy required to remove an electron from a single atom/molecule is called the ionization energy.
Ionization can be accomplished by adding heat but there are other alternatives.
e.g. shining light, and collisions with energetic particles.
Like other phase changes, the temperature remains constant during the phase change.
The ionization temperature of hydrogen gas is ~10,000 K.
When electrons are captured by ions the process is called recombination.
Recombination is the opposite of ionization.
Usually, the energy released by recombination is emitted in the form of light.
Special Points in a Phase Diagram
There are two types of special points in a phase diagram: triple points and critical points.

Triple Points
For every substance that can exist in three phases (e.g. as a solid, liquid and a gas) there is a unique temperature and pressure at which all three phases can coexist.
This combination of temperature and pressure is called the substance’s triple point.
You can have triple points between any three phases of the substance e.g. two solid phases and the gas.
Helium has a triple point between two liquid phases and the gas.
The triple point of pure water used to be the second fixed point on the Kelvin temperature scale.
The temperature of the triple point of water was defined to be 273.16 K
Critical Points
For every substance that can exist as a liquid and a gas, there is a unique temperature and pressure at which the distinction between liquid and gas disappears.
This combination of temperature and pressure is called the substance’s critical point.
You can also have critical points between any other two phases of the substance e.g. two solid phases or two liquid phases
For temperatures above the critical point, gas cannot be liquefied by pressure alone.
A substance with a temperature and pressure larger than the liquid-vapor critical point is sometimes called a supercritical fluid.
The critical point of pure water is 373.946 °C and 22,060 kPa.
Supercritical water can be found around ‘black smokers’, geothermally heated water issuing from vents on the sea floor.
Superheating/Supercooling
Phase changes don’t occur uniformly in a substance, they are usually patchy e.g. when a liquid boils it forms bubbles.
Bubbles in a boiling liquid often form around imperfections of some kind which form nucleation sites.
Bubbles start small and then grow (they expand and the amount of gas in the bubble increases due to evaporation from the bubble surface)
The gas pressure must overcome the liquid pressure and also overcome the surface tension of the liquid.
Because a small bubble has a large surface-to-volume ratio, it takes a larger gas pressure to make a small bubble than a big bubble.
If small bubbles cannot form, the liquid does not undergo the phase transition and can be heated to a temperature higher than the boiling point.
Such a liquid is called superheated.
Superheated liquid hydrogen was once used in high-energy particle detectors known as ‘bubble chambers’.
Particles traveling through the superheated liquid create nucleation sites which then form bubbles.
Photographs of the bubbles reveals the path of the particle.
A similar phenomenon can occur when a gas tries to condense into a liquid or when liquids try to freeze into solids.
Without nucleation sites the gas can be cooled below the boiling temperature or the liquid below its freezing temperature.
Such a gas / liquid is called supercooled.
Pure water can be supercooled to −48.3 °C.
Supercooled carbon dioxide gas was once used in high energy particle detectors known as ‘cloud chambers’.
 Knowt
Knowt
