
Chapter 11 - Liquids and Intermolecular
Intermolecular - Forces between molecules.
Boiling breaks intermolecular forces.
Properties that reflect intermolecular forces
Boiling point
Melting point
Viscosity
Surface tension
Capillary action
Water has a high surface tension.
States of matter
Gas
Liquid
Solid
Gases and liquids are called fluids.
Liquids and solids are called condensed states, they have strong forces.
Intermolecular forces (Weakest to strongest)
Dispersion forces or London forces - Only occurs in non-polar molecules. It is constantly shifting to a different set of temporary forces.
The tighter the molecules, the lower the surface area they have, and the lower their boiling point is.
Ex. Neopentane has a lower boiling point than pentane because neopentane has a lower surface area.
Dipole-dipole interactions - They form permanent dipoles. Occurs in polar molecules.
Bad interactions - 2 positive or 2 negative banging into each other.
Hydrogen bonding - Strongest force. It can only be formed with a hydrogen atom bonding with a nitrogen, oxygen, or fluorine atom.
Crystalatus - Smallest defined unit that repeats inside a molecule.
Water is the only liquid that freezes from the top.
Ion-dipole interactions
Found in solutions of ions.
Can only occur with polar compounds.
Relative strengths of intermolecular forces
When 2 molecules have comparable moral masses and shapes, dispersion forces are equal.
When 2 molecules have very different molar masses and there's no H-bonding, dispersion force determines the substance with stronger attractions.
Properties affected by intermolecular forces
Viscosity - Resistance of a liquid to flow. Increases with strong forces, decreases with higher temperature.
Surface tension - Water acts as if it has a skin be of the extra forces on the surface allowing water to bead up when in contact with nonpolar surfaces.
Capillary action - The rise of liquid up narrow tubes.
Cohesive forces - Intermolecular forces that bind similar molecules to one another.
Adhesive forces - Intermolecular forces that bind a substance to a surface.
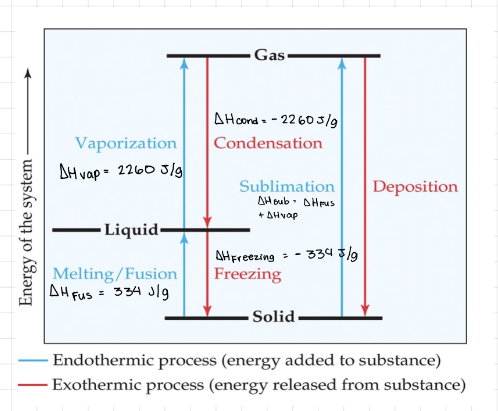
Phase changes
Phase change - Conversion from one state to matter to another.
Melting / Fusion - Solid to liquid, endothermic.
Freezing - Liquid to solid, exothermic.
Vaporization - Liquid to gas, endothermic.
Condensation - Gas to liquid, exothermic.
Sublimation - Solid to gas, endothermic.
Deposition - Gas to solid, exothermic.
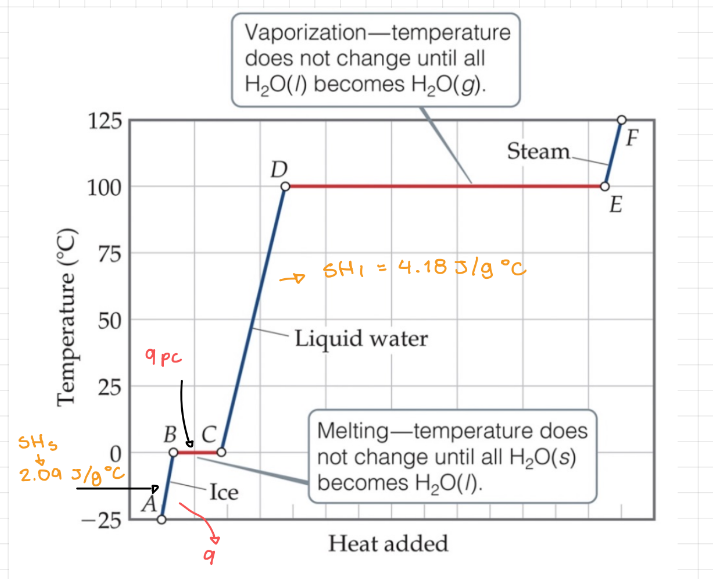
Heating Curves
Heating curve - Graph of temperature (y) and the heat added (x).
Vapor pressure
As temperature increases, more molecules are able to have enough energy to become a gas.
P = nRT/V
P = MRT
M - molarity
R - gas constant
Vapor pressure - How much of a liquid evaporates at a certain pressure.
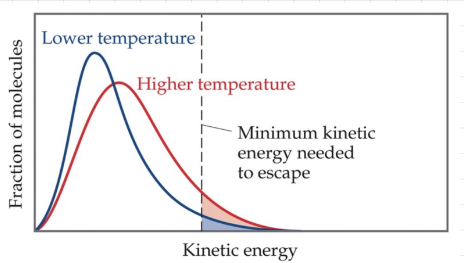
At any temperature, some liquid molecules have enough energy to escape the surface and become a gas.
Vapor pressure curves

Natural log of the vapor pressure - It’s inversely proportional to its temperature.
Clausius-Clayperon equation

We can find ΔH of vaporization if we know the vapor pressure and the temperature at one point.
We can find the pressure at point 1 when we know the ΔH of vaporization and the temperature at point 2.
Formula simplification

Phase diagrams
Phase diagram - A graph that shows the states of matter under conditions of temperature and pressure.
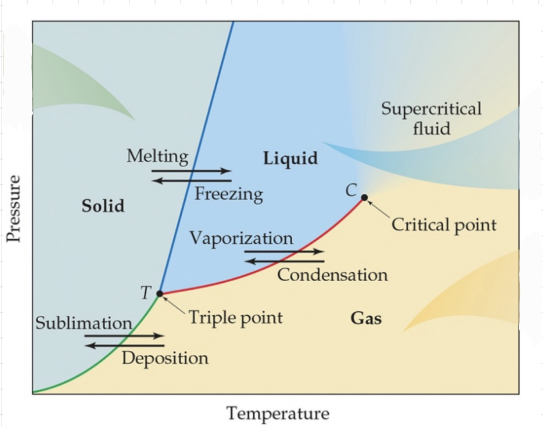
Triple point - The point where all three states of matter coexist.
Critical point - The point at which no amount of pressure alone can liquify the gas.
Here you can’t tell the difference between a gas and a liquid.
PHASE DIAGRAM OF WATER

Water can melt only by pressure.
The slope of the melting curve is negative, meaning that as the pressure goes up, the melting point goes down.
CARBON PHASE DIAGRAM
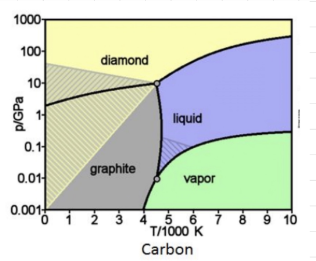
Carbon has two triple points.
Triple points are always between the liquid and gas or between two solids.
Chapter 11 - Liquids and Intermolecular
Intermolecular - Forces between molecules.
Boiling breaks intermolecular forces.
Properties that reflect intermolecular forces
Boiling point
Melting point
Viscosity
Surface tension
Capillary action
Water has a high surface tension.
States of matter
Gas
Liquid
Solid
Gases and liquids are called fluids.
Liquids and solids are called condensed states, they have strong forces.
Intermolecular forces (Weakest to strongest)
Dispersion forces or London forces - Only occurs in non-polar molecules. It is constantly shifting to a different set of temporary forces.
The tighter the molecules, the lower the surface area they have, and the lower their boiling point is.
Ex. Neopentane has a lower boiling point than pentane because neopentane has a lower surface area.
Dipole-dipole interactions - They form permanent dipoles. Occurs in polar molecules.
Bad interactions - 2 positive or 2 negative banging into each other.
Hydrogen bonding - Strongest force. It can only be formed with a hydrogen atom bonding with a nitrogen, oxygen, or fluorine atom.
Crystalatus - Smallest defined unit that repeats inside a molecule.
Water is the only liquid that freezes from the top.
Ion-dipole interactions
Found in solutions of ions.
Can only occur with polar compounds.
Relative strengths of intermolecular forces
When 2 molecules have comparable moral masses and shapes, dispersion forces are equal.
When 2 molecules have very different molar masses and there's no H-bonding, dispersion force determines the substance with stronger attractions.
Properties affected by intermolecular forces
Viscosity - Resistance of a liquid to flow. Increases with strong forces, decreases with higher temperature.
Surface tension - Water acts as if it has a skin be of the extra forces on the surface allowing water to bead up when in contact with nonpolar surfaces.
Capillary action - The rise of liquid up narrow tubes.
Cohesive forces - Intermolecular forces that bind similar molecules to one another.
Adhesive forces - Intermolecular forces that bind a substance to a surface.
Phase changes
Phase change - Conversion from one state to matter to another.
Melting / Fusion - Solid to liquid, endothermic.
Freezing - Liquid to solid, exothermic.
Vaporization - Liquid to gas, endothermic.
Condensation - Gas to liquid, exothermic.
Sublimation - Solid to gas, endothermic.
Deposition - Gas to solid, exothermic.

Heating Curves
Heating curve - Graph of temperature (y) and the heat added (x).

Vapor pressure
As temperature increases, more molecules are able to have enough energy to become a gas.
P = nRT/V
P = MRT
M - molarity
R - gas constant
Vapor pressure - How much of a liquid evaporates at a certain pressure.

At any temperature, some liquid molecules have enough energy to escape the surface and become a gas.
Vapor pressure curves

Natural log of the vapor pressure - It’s inversely proportional to its temperature.
Clausius-Clayperon equation

We can find ΔH of vaporization if we know the vapor pressure and the temperature at one point.
We can find the pressure at point 1 when we know the ΔH of vaporization and the temperature at point 2.
Formula simplification

Phase diagrams
Phase diagram - A graph that shows the states of matter under conditions of temperature and pressure.

Triple point - The point where all three states of matter coexist.
Critical point - The point at which no amount of pressure alone can liquify the gas.
Here you can’t tell the difference between a gas and a liquid.
PHASE DIAGRAM OF WATER

Water can melt only by pressure.
The slope of the melting curve is negative, meaning that as the pressure goes up, the melting point goes down.
CARBON PHASE DIAGRAM

Carbon has two triple points.
Triple points are always between the liquid and gas or between two solids.
 Knowt
Knowt
