
CHEMISTRY: CHEMICAL REACTIONS AND EQUATIONS
Chemical Reactions and Equations
How do we use chemical symbols to form equations that represent these chemical changes?
Physical change: change in appearance of the starting material
Chemical change: change of properties between the product and reactants
A well-defined chemical change is called a chemical reaction
Chemical equations
A chemical equation shows what takes place during a chemical reaction.
A + sign is used to separate each of the reactants and each of the products. The arrow is read as “produces” or “yields”.
A substance that enters into a reaction is called a reactant and is written to the left of the arrow
A substance that is produced by a reaction is called a productand is written to the right of the arrow. 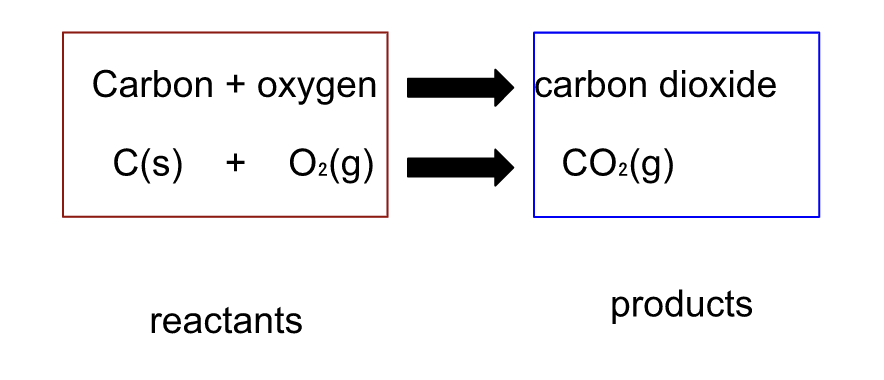
Endothermic and Exothermic Processes
Summary of Endothermic and Exothermic Processes |
|---|
Type of reaction | Surrounding temperature | Potential energy of reactants | Potential energy of products | Energy change |
|---|---|---|---|---|
Endothermic | decreases | less | more | Positive |
Exothermic | increases | more | less | negative |
Endothermic processes
Endothermic processes: processes that require energy in order to occur.
Endothermic changes include: The physical change of ice melting, food cooking, and evaporating liquid water.
Reactants absorb energy as they become products, hence the products have more potential energy than the reactions.
Also lowering temperature as it absorbs from surroundings.
Exothermic processes
Exothermic: processes that release thermal energy when they occur.
Examples include: the burning of carbon in oxygen and freezing of water.
Energy released from these processes is given off to the surroundings, thus raising the surrounding temperature.
In exothermic reactions, the products have less potential than the reactants.
Balancing chemical equations
In a correctly written chemical equation the number of each type of atom is the same on both sides of the equation.
This observation relates to the law of conservation of mass, which states that matter is neither created nor destroyed in chemical reactions.
In any chemical reaction and numbers and kinds of atoms must remain unchanged in the reaction
Balanced Chemical Equation : C + O₂ → CO₂
Unbalanced Chemical Equation: H₂ + O₂ → H₂O
Observe the equations above.
The first equation shows the conservation of atoms and agrees with the law of conservation of mass. There is one carbon atom and two oxygen atoms on both of the equation. Therefore this equation is balanced.
However, the second equation is different. While there are two atoms of hydrogen on both sides of the arrow, there are two atoms of oxygen on the reactant side but only one atom of oxygen on the product side. This equation is not balanced.
So how must the equation be changed, so that it is balanced?
We balance equations by changing the coefficients in the equation once correct formulas have been written.
Since we cannot remove atoms, the only way to balance the equation would be to add more atoms
H₂ + O₂ → H₂O (our unbalanced equation)
Since there is only 1 oxygen atom on the right side, we can place a coefficient of 2 in front of the formula for water to balance out oxygen atoms.
H₂ + O₂ → 2H₂O
Coefficients apply to the whole water formula, which means although we have a balance of oxygen atoms, the hydrogen atoms have now become unbalanced.
2H₂ + O₂ → 2H₂O
This can be solved by placing a 2 in front of the formula for hydrogen.
Having 4 hydrogen and 2 oxygen atoms, our chemical equation has now been balanced.
Common notation used in Equations |
|---|
Symbol | Meaning |
|---|---|
+ | Separates two reactants or two products |
→ | Separates reactants from products |
(s) | Identifies substance as a solid |
(l) | Identifies substance as a liquid |
(g) | Identifies the substance as a gas |
(aq) | Identifies the substance as being dissolved in aqueous(water) solution |
CHEMISTRY: CHEMICAL REACTIONS AND EQUATIONS
Chemical Reactions and Equations
How do we use chemical symbols to form equations that represent these chemical changes?
Physical change: change in appearance of the starting material
Chemical change: change of properties between the product and reactants
A well-defined chemical change is called a chemical reaction
Chemical equations
A chemical equation shows what takes place during a chemical reaction.
A + sign is used to separate each of the reactants and each of the products. The arrow is read as “produces” or “yields”.
A substance that enters into a reaction is called a reactant and is written to the left of the arrow
A substance that is produced by a reaction is called a productand is written to the right of the arrow. 
Endothermic and Exothermic Processes
Summary of Endothermic and Exothermic Processes |
|---|
Type of reaction | Surrounding temperature | Potential energy of reactants | Potential energy of products | Energy change |
|---|---|---|---|---|
Endothermic | decreases | less | more | Positive |
Exothermic | increases | more | less | negative |
Endothermic processes
Endothermic processes: processes that require energy in order to occur.
Endothermic changes include: The physical change of ice melting, food cooking, and evaporating liquid water.
Reactants absorb energy as they become products, hence the products have more potential energy than the reactions.
Also lowering temperature as it absorbs from surroundings.
Exothermic processes
Exothermic: processes that release thermal energy when they occur.
Examples include: the burning of carbon in oxygen and freezing of water.
Energy released from these processes is given off to the surroundings, thus raising the surrounding temperature.
In exothermic reactions, the products have less potential than the reactants.
Balancing chemical equations
In a correctly written chemical equation the number of each type of atom is the same on both sides of the equation.
This observation relates to the law of conservation of mass, which states that matter is neither created nor destroyed in chemical reactions.
In any chemical reaction and numbers and kinds of atoms must remain unchanged in the reaction
Balanced Chemical Equation : C + O₂ → CO₂
Unbalanced Chemical Equation: H₂ + O₂ → H₂O
Observe the equations above.
The first equation shows the conservation of atoms and agrees with the law of conservation of mass. There is one carbon atom and two oxygen atoms on both of the equation. Therefore this equation is balanced.
However, the second equation is different. While there are two atoms of hydrogen on both sides of the arrow, there are two atoms of oxygen on the reactant side but only one atom of oxygen on the product side. This equation is not balanced.
So how must the equation be changed, so that it is balanced?
We balance equations by changing the coefficients in the equation once correct formulas have been written.
Since we cannot remove atoms, the only way to balance the equation would be to add more atoms
H₂ + O₂ → H₂O (our unbalanced equation)
Since there is only 1 oxygen atom on the right side, we can place a coefficient of 2 in front of the formula for water to balance out oxygen atoms.
H₂ + O₂ → 2H₂O
Coefficients apply to the whole water formula, which means although we have a balance of oxygen atoms, the hydrogen atoms have now become unbalanced.
2H₂ + O₂ → 2H₂O
This can be solved by placing a 2 in front of the formula for hydrogen.
Having 4 hydrogen and 2 oxygen atoms, our chemical equation has now been balanced.
Common notation used in Equations |
|---|
Symbol | Meaning |
|---|---|
+ | Separates two reactants or two products |
→ | Separates reactants from products |
(s) | Identifies substance as a solid |
(l) | Identifies substance as a liquid |
(g) | Identifies the substance as a gas |
(aq) | Identifies the substance as being dissolved in aqueous(water) solution |
 Knowt
Knowt
