
Photosynthesis AP BIO
Energy 3
Cellular Energetics
https://docs.google.com/document/d/12Pey85sa8sMjp_32rvz9QtyL1ClU8o8_4_k93tdQPPo/edit
Theory
2 Relevant Laws
First
Matter and energy cannot be created or destroyed
transformations are allowed
Work: anything that requires atoms to be moved around through cellular actions
Kinetic and Potential Energy
Gibbs Free Energy
Exergonic Reactions:
Release energy (matter is converted from higher energy arrangements to lower energy arrangements)
Will happen spontaneously. once they are initiated
Change in free energy is Negative
Endergonic Reactions
Require energy input to occur (matter is converted from lower energy arrangements to higher energy arrangements)
Can not occur spontaneously
Change in free energy is Positive
Biological Systems use exergonic reactions to provide the free energy necessary for endergonic reactions
living systems are not the only systems in the universe that require energy conversion to function
Second
Any closed system will tend toward a state of maximum entropy
True for the Universe as a whole
Portions of the Universe can still function as “Open”systems
energy (and the matter that accompanies it) can be used to decrease an open systemś entropy
Life is Highly ordered
Organisms use the energy they convert to power cellular/organism processes that decrease their overall entropy (or at least dealy its increase)
This process increases the entropy of their surroundings
Life requires energy input
A highly ordered living system uses energy input to maintain/increase order
Open & Closed Systems
Closed
Closed systems inexorably tend towards an absence of free energy
They reach at a state of equilibrium between inputs and outputs
Inevitably Dull
Open
Open systems will not reach equilibrium as long as the processes of the system receive inputs and produce outputs
There is no Inherent limit to the complexity of an open system, provided there is enough input to allow for that complexity
Usually interesting
Life is an open system
Equilibrium = Death
Cellular Energy Theory
ATP
Adenosine Triphosphate
The short term energy storage/release molecule of choice in cells
Tens of millions made and used per second per cell
The bonds between phosphate groups in nucleoside triphosphates (like ATP) are relatively unstable
Much more free energy is released when the bonds between them are broken than is required by the cell to initiate their cleavage
Much of the work done by cellular proteins is mediated by the addition and removal of phosphate groups from ATP by proteins to other proteins (kinases and phosphatases)
Metabolism
refers to the sum total of all chemical reactions that take place in an organism
Energy from catabolic reactions (ex. respiration) is used to power the synthesis of ATP from ADP and Phosphate groups
ATP ( and other NTP’s) is used to power the anabolic Reactions that Require chemical energy
Reaction Coupling
Refers to linked an exergonic process with a cellular process
If an endergonic process requires less free energy than an exergonic process produces, coupling those two reactions allow for maximum efficacy , and an overall negative delta G
The Return of Kinetics
The Reaction Profile
All reactions require an input of energy (the “activation energy”) to make the breaking of current chemical bonds energetically favorable (the “transition state”)
The relationship between the energy of the products and the energy of the reactants is what determines if a reaction is exergonic or endergonic
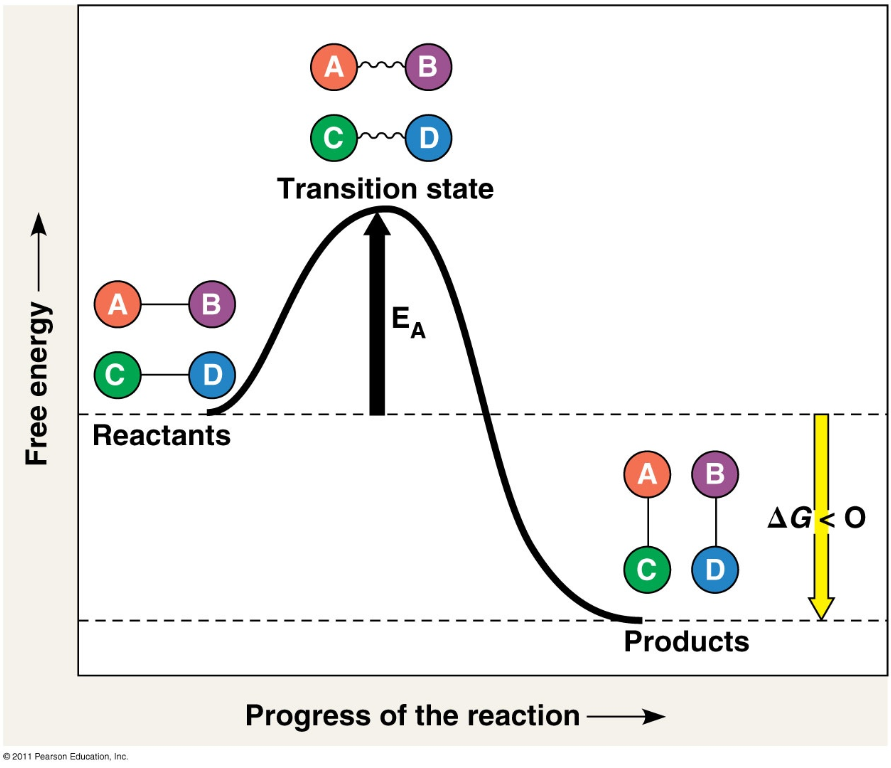
Catalysts
Any substance that increases the rate of a chemical reaction while not participating in the reaction
Lowers the activation energy of a reaction
Reusable (since they don’t participate)
Photoautotrophic Nutrition
https://docs.google.com/document/d/1qmyeIgr29kYODB3fmjEbZEs4OPSSfc-r7SiRSI6E9S0/edit
Photoautotrophic Energy Processing
Photosynthesis
A Quick Recap
Light Makes Us Food
without light out wouldn’t be able to eat anything
And you want to eat don’t you
There is a reciprocal relationship between chemoheterotrophic nutrition and photoautotrophic nutrition
The inputs of one are the outputs of the other
This account for this curious fact
Photosynthesis
6C|O2 + 6|H2O = C6|H12|O6 + 6|O2
Aerobic Cellular Respiration
C6|H12|O6 + 6|O2 = 6|CO2 + 6H|2O
Plants and Such
Remember plants are NOT the only photoautotrophs
Most of the Oxygen in the atmosphere is generated by cyanobacteria and aquatic protists
Plant Anatomy photosynthesis happens at the leaves, organs which are specialized for the process
At the leaf, mesophyll cells are full of chloroplasts, the site of photosynthesis
Light = Energy
Light is a form of electromagnetic radiation
it is produced by the movement of electrons between orbitals
visible light is just one tiny slice of the larger electromagnetic spectrum
Chlorophyll
Chlorophyll is a pigment
Pigment: any molecule that interacts with light energy to produce a color
Chlorophyll comes in two main varieties
Chlorophyll A
Chlorophyll B
While chlorophyll is the main photosynthetic pigment, it is NOt the only pigment found chloroplasts
Accessory Pigments
Other pigments that allow the chloroplast to absorb a wider range of light, and protect the chloroplasts from light-related damage
carotenoids
xanthophylls
Why are plants green?
sunlight contains almost all wavelengths of visible light
Chloroplasts do not absorb all wavelengths of light equally
WHen plants are exposed to Light, chloroplast preferentially absorb light in the blue and red portions of the visible light spectrum
The accessory pigments expand the useful range of light (the “action spectrum”) but green is still the least useful
The unequal utility of different wavelengths of light was noticed by Theodore Engelmann. who observed higher rates of growth of aerobic bacteria on algae grown in blue and red wavelengths of light
Light
an anabolic, endergonic process
water will be oxidized (it is the reduction agent)
Carbon will be reduced (it is the oxidizing agent)
Chloroplast
Chloroplast consists of a series of membranous disks (thylakoids) arranged in stacks (grana)
The grana are inside of the inner membrane of the chloroplast
The fluid/space surrounding the grana is called the stroma
Photosynthetic prokaryotes use specialized cell membrane region to accomplish photosynthesis
Photosynthesis is a 2-part process
The light reactions
Occur in the thylakoid membranes
Light is used to drive the production of ATP and NADPH (electron shuttle)
Water provided the electrons needed and is converted to oxygen gas (a waste product)
The Calvin Cycle
Occurs in the stoma
The ATP and NADPH produced in the light reactions are used to incorporate carbon dioxide into a 3-carbon sugar
The Light Reactions
Light + Chlorophyll = Electrons!
When photon of light interact with chlorophyll, electrons in the Magnesium atom become excited
This happens with -1% of all sunlight that strikes the surface of the earth
Isolated chlorophyll will fluoresce when exposed to light, as the excited electrons return to the ground state
Photosystems
complexes of protein and pigment molecules that are embedded in the thylakoid membrane
Direct incoming photons into the “reaction center” where chlorophyll a molecules produce excited electrons which are transferred to an electron transport chain
Two types
Photosystem II
center chlorophyll works best at a light wavelength of 680 nm (P680)
found at the “start” of the ETC
Photosystem I
central chlorophyll works best at a light wavelength of 700 nm (P&00)
found at the “end” of the ETC
Since chlorophyll is not going to have the electrons return to it, new electrons are needed
Water provides the replacement electrons “photolysis” This creates 4 protons and 1 molecule of oxygen gas for every 2 water molecules consumed
The oxygen gas is released as a waste product, becoming a major input for aerobic cellular respiration
Chemiosmosis
As the electrons move through the ETC, they provide the energy for chemiosmosis, in a fashion almost identical to in cellular respiration
Protons are pumped by ETC proteins from the stoma into the thylakoid space
The facilitated diffusion of protons back into the stroma through ATP synthase drives the synthesis of ATP
One notable difference
In respiration, the energy comes from oxidation of glucose “oxidative phosphorylation” In photosynthesis, the energy comes from photons “photophosphorylation”
Electron Flow
Non-Cyclic Electron Flow
Electrons move from photosystem II to photosystem I via ETC
From photosystem II, they are transferred to the enzyme NADP-Reductase which uses them to reduce NADP+ into NADPH
Produces both ATP and NADPH
Requires water
Cyclic Electron Flow
Electrons move from photosystem I to ETC before returning to photosystem I
Only produces ATP
Does not require water
Why?
The Calvin cycle will require 9 ATP and ^ NADPH for every sugar produced
Fun Fact
Even if you are growing plants indoors, you are still using sunlight to do it, just a version that was stored in the chemical bonds of the fossil fuels that are being used to power the electric lights
Inputs
Light
ADP + Pi
NADP+
Water
Outputs
ATP
NADPH
O2
Calvin Cycle
Three Phases
Carbon Fixation
Ribulose Bisphosphate Carboxylase (aka “RuBisCo”) mediates the transfer of a molecule of Carbon Dioxide onto a molecule of Ribulose Bisphosphate (RuBP - 5 C)
Reduction
ATP and NADPH are used to rearrange RUBP into Glyceraldehyde 3-phosphate (G3P, aka PGAL) a three-carbon sugar
Regeneration
ATP is used to reconstitute RuBP from G3P
Where’s the Sugar?
In order to get 1 G3P as a product of the Calvin Cycle, 3 molecules of carbon dioxide have to be joined to three molecules of RuBP
This makes 6 molecules of G3P, 1 of which is a net product
The other 5 G3P are used to regenerate three molecules of RuBP
G3P is a sugar building block. 2 G3P can make 1 6 carbon sugar. Many G3P can make a polysaccharide
Fun Fact
The Calvin cycle is named for Melvin Calvin who discovered it by using radioactive C-14 to trace the path of Carbon through the cycle
He received a Nobel Prize for his efforts in 1961
It is also commonly referred to as simply “Carbon Fixation”
It is never, ever, call, “The Dark Reaction”
Inputs (per G3P) {x2 to make glucose}
3 CO2
9 ATP
6 NADPH
Outputs
1 G3P
9 ADP + Pi
6 NADP+
Versatility and Regulation
An evolutionary quirk
Rubisco evolved in conditions of low oxygen gas concentration
As a result, its active site has a high affinity for oxygen gas
Which is a problem
Photorespiration
The metabolic pathways that occur when rubisco incorporates Oxygen instead of Carbon Dioxide into RuBP
A metabolic dead end. Used ATP but produces no sugar
Best if avoided
Lots of times, not a problem
As long as a plant can keep its stomata open and exchange gas with the environment, photorespiration is kept to a minimum
C3 Leaves
No adaptations for minimizing photorespiration
Both stages of photosynthesis occur in the same cell simultaneously
Oxygen and Carbon Dioxide are exchanged with the environment through the stomata
Sugars are transported to vascular tissue for transport throughout the plant
But there are environments where keeping stomata open will lead to desiccation
Closed stomata
increasing [oxygen gas]/decarseing [carbon dioxide]
increased photorespiration
NOT GOOD
2 major adaptations
C4 Leaves (separation)
Spatial Separation
Carbon fixation occurs in mesophyll cells
Carbon dioxide is incorporated into 4C organic acid (malate) by the enzyme PEP carboxylase
The 4C acid is then transported to bundle sheath cells, where the carbon dioxide is cleaved from the 4C acid
Since the bundle sheath cells are surrounded by mesophyll, their oxygen gas concentration remains low, even as the light reaction occurs in mesophyll cells
CAM Plants
Carbon fixation occurs during the evening when open stomates will not lead to desiccation
The carbon dioxide is stored in an organic acid
During the day , the organic acid store is used to supply the calvin cycle with carbon dioxide
Importance
Well…
Consider everyone you know, ever pet you have ever had, every ancestor in your lineage…they have all been able to exist for the simple fact that photoautotrophs make more food than they need and produce oxygen gas as a waste product
Also…
Modern industry is more and more interested in using plants to do all sorts of things (like make biofuels, for instance)
Photosynthesis AP BIO
Energy 3
Cellular Energetics
https://docs.google.com/document/d/12Pey85sa8sMjp_32rvz9QtyL1ClU8o8_4_k93tdQPPo/edit
Theory
2 Relevant Laws
First
Matter and energy cannot be created or destroyed
transformations are allowed
Work: anything that requires atoms to be moved around through cellular actions
Kinetic and Potential Energy
Gibbs Free Energy
Exergonic Reactions:
Release energy (matter is converted from higher energy arrangements to lower energy arrangements)
Will happen spontaneously. once they are initiated
Change in free energy is Negative
Endergonic Reactions
Require energy input to occur (matter is converted from lower energy arrangements to higher energy arrangements)
Can not occur spontaneously
Change in free energy is Positive
Biological Systems use exergonic reactions to provide the free energy necessary for endergonic reactions
living systems are not the only systems in the universe that require energy conversion to function
Second
Any closed system will tend toward a state of maximum entropy
True for the Universe as a whole
Portions of the Universe can still function as “Open”systems
energy (and the matter that accompanies it) can be used to decrease an open systemś entropy
Life is Highly ordered
Organisms use the energy they convert to power cellular/organism processes that decrease their overall entropy (or at least dealy its increase)
This process increases the entropy of their surroundings
Life requires energy input
A highly ordered living system uses energy input to maintain/increase order
Open & Closed Systems
Closed
Closed systems inexorably tend towards an absence of free energy
They reach at a state of equilibrium between inputs and outputs
Inevitably Dull
Open
Open systems will not reach equilibrium as long as the processes of the system receive inputs and produce outputs
There is no Inherent limit to the complexity of an open system, provided there is enough input to allow for that complexity
Usually interesting
Life is an open system
Equilibrium = Death
Cellular Energy Theory
ATP
Adenosine Triphosphate
The short term energy storage/release molecule of choice in cells
Tens of millions made and used per second per cell
The bonds between phosphate groups in nucleoside triphosphates (like ATP) are relatively unstable
Much more free energy is released when the bonds between them are broken than is required by the cell to initiate their cleavage
Much of the work done by cellular proteins is mediated by the addition and removal of phosphate groups from ATP by proteins to other proteins (kinases and phosphatases)
Metabolism
refers to the sum total of all chemical reactions that take place in an organism
Energy from catabolic reactions (ex. respiration) is used to power the synthesis of ATP from ADP and Phosphate groups
ATP ( and other NTP’s) is used to power the anabolic Reactions that Require chemical energy
Reaction Coupling
Refers to linked an exergonic process with a cellular process
If an endergonic process requires less free energy than an exergonic process produces, coupling those two reactions allow for maximum efficacy , and an overall negative delta G
The Return of Kinetics
The Reaction Profile
All reactions require an input of energy (the “activation energy”) to make the breaking of current chemical bonds energetically favorable (the “transition state”)
The relationship between the energy of the products and the energy of the reactants is what determines if a reaction is exergonic or endergonic

Catalysts
Any substance that increases the rate of a chemical reaction while not participating in the reaction
Lowers the activation energy of a reaction
Reusable (since they don’t participate)
Photoautotrophic Nutrition
https://docs.google.com/document/d/1qmyeIgr29kYODB3fmjEbZEs4OPSSfc-r7SiRSI6E9S0/edit
Photoautotrophic Energy Processing
Photosynthesis
A Quick Recap
Light Makes Us Food
without light out wouldn’t be able to eat anything
And you want to eat don’t you
There is a reciprocal relationship between chemoheterotrophic nutrition and photoautotrophic nutrition
The inputs of one are the outputs of the other
This account for this curious fact
Photosynthesis
6C|O2 + 6|H2O = C6|H12|O6 + 6|O2
Aerobic Cellular Respiration
C6|H12|O6 + 6|O2 = 6|CO2 + 6H|2O
Plants and Such
Remember plants are NOT the only photoautotrophs
Most of the Oxygen in the atmosphere is generated by cyanobacteria and aquatic protists
Plant Anatomy photosynthesis happens at the leaves, organs which are specialized for the process
At the leaf, mesophyll cells are full of chloroplasts, the site of photosynthesis
Light = Energy
Light is a form of electromagnetic radiation
it is produced by the movement of electrons between orbitals
visible light is just one tiny slice of the larger electromagnetic spectrum
Chlorophyll
Chlorophyll is a pigment
Pigment: any molecule that interacts with light energy to produce a color
Chlorophyll comes in two main varieties
Chlorophyll A
Chlorophyll B
While chlorophyll is the main photosynthetic pigment, it is NOt the only pigment found chloroplasts
Accessory Pigments
Other pigments that allow the chloroplast to absorb a wider range of light, and protect the chloroplasts from light-related damage
carotenoids
xanthophylls
Why are plants green?
sunlight contains almost all wavelengths of visible light
Chloroplasts do not absorb all wavelengths of light equally
WHen plants are exposed to Light, chloroplast preferentially absorb light in the blue and red portions of the visible light spectrum
The accessory pigments expand the useful range of light (the “action spectrum”) but green is still the least useful
The unequal utility of different wavelengths of light was noticed by Theodore Engelmann. who observed higher rates of growth of aerobic bacteria on algae grown in blue and red wavelengths of light
Light
an anabolic, endergonic process
water will be oxidized (it is the reduction agent)
Carbon will be reduced (it is the oxidizing agent)
Chloroplast
Chloroplast consists of a series of membranous disks (thylakoids) arranged in stacks (grana)
The grana are inside of the inner membrane of the chloroplast
The fluid/space surrounding the grana is called the stroma
Photosynthetic prokaryotes use specialized cell membrane region to accomplish photosynthesis
Photosynthesis is a 2-part process
The light reactions
Occur in the thylakoid membranes
Light is used to drive the production of ATP and NADPH (electron shuttle)
Water provided the electrons needed and is converted to oxygen gas (a waste product)
The Calvin Cycle
Occurs in the stoma
The ATP and NADPH produced in the light reactions are used to incorporate carbon dioxide into a 3-carbon sugar
The Light Reactions
Light + Chlorophyll = Electrons!
When photon of light interact with chlorophyll, electrons in the Magnesium atom become excited
This happens with -1% of all sunlight that strikes the surface of the earth
Isolated chlorophyll will fluoresce when exposed to light, as the excited electrons return to the ground state
Photosystems
complexes of protein and pigment molecules that are embedded in the thylakoid membrane
Direct incoming photons into the “reaction center” where chlorophyll a molecules produce excited electrons which are transferred to an electron transport chain
Two types
Photosystem II
center chlorophyll works best at a light wavelength of 680 nm (P680)
found at the “start” of the ETC
Photosystem I
central chlorophyll works best at a light wavelength of 700 nm (P&00)
found at the “end” of the ETC
Since chlorophyll is not going to have the electrons return to it, new electrons are needed
Water provides the replacement electrons “photolysis” This creates 4 protons and 1 molecule of oxygen gas for every 2 water molecules consumed
The oxygen gas is released as a waste product, becoming a major input for aerobic cellular respiration
Chemiosmosis
As the electrons move through the ETC, they provide the energy for chemiosmosis, in a fashion almost identical to in cellular respiration
Protons are pumped by ETC proteins from the stoma into the thylakoid space
The facilitated diffusion of protons back into the stroma through ATP synthase drives the synthesis of ATP
One notable difference
In respiration, the energy comes from oxidation of glucose “oxidative phosphorylation” In photosynthesis, the energy comes from photons “photophosphorylation”
Electron Flow
Non-Cyclic Electron Flow
Electrons move from photosystem II to photosystem I via ETC
From photosystem II, they are transferred to the enzyme NADP-Reductase which uses them to reduce NADP+ into NADPH
Produces both ATP and NADPH
Requires water
Cyclic Electron Flow
Electrons move from photosystem I to ETC before returning to photosystem I
Only produces ATP
Does not require water
Why?
The Calvin cycle will require 9 ATP and ^ NADPH for every sugar produced
Fun Fact
Even if you are growing plants indoors, you are still using sunlight to do it, just a version that was stored in the chemical bonds of the fossil fuels that are being used to power the electric lights
Inputs
Light
ADP + Pi
NADP+
Water
Outputs
ATP
NADPH
O2
Calvin Cycle
Three Phases
Carbon Fixation
Ribulose Bisphosphate Carboxylase (aka “RuBisCo”) mediates the transfer of a molecule of Carbon Dioxide onto a molecule of Ribulose Bisphosphate (RuBP - 5 C)
Reduction
ATP and NADPH are used to rearrange RUBP into Glyceraldehyde 3-phosphate (G3P, aka PGAL) a three-carbon sugar
Regeneration
ATP is used to reconstitute RuBP from G3P
Where’s the Sugar?
In order to get 1 G3P as a product of the Calvin Cycle, 3 molecules of carbon dioxide have to be joined to three molecules of RuBP
This makes 6 molecules of G3P, 1 of which is a net product
The other 5 G3P are used to regenerate three molecules of RuBP
G3P is a sugar building block. 2 G3P can make 1 6 carbon sugar. Many G3P can make a polysaccharide
Fun Fact
The Calvin cycle is named for Melvin Calvin who discovered it by using radioactive C-14 to trace the path of Carbon through the cycle
He received a Nobel Prize for his efforts in 1961
It is also commonly referred to as simply “Carbon Fixation”
It is never, ever, call, “The Dark Reaction”
Inputs (per G3P) {x2 to make glucose}
3 CO2
9 ATP
6 NADPH
Outputs
1 G3P
9 ADP + Pi
6 NADP+
Versatility and Regulation
An evolutionary quirk
Rubisco evolved in conditions of low oxygen gas concentration
As a result, its active site has a high affinity for oxygen gas
Which is a problem
Photorespiration
The metabolic pathways that occur when rubisco incorporates Oxygen instead of Carbon Dioxide into RuBP
A metabolic dead end. Used ATP but produces no sugar
Best if avoided
Lots of times, not a problem
As long as a plant can keep its stomata open and exchange gas with the environment, photorespiration is kept to a minimum
C3 Leaves
No adaptations for minimizing photorespiration
Both stages of photosynthesis occur in the same cell simultaneously
Oxygen and Carbon Dioxide are exchanged with the environment through the stomata
Sugars are transported to vascular tissue for transport throughout the plant
But there are environments where keeping stomata open will lead to desiccation
Closed stomata
increasing [oxygen gas]/decarseing [carbon dioxide]
increased photorespiration
NOT GOOD
2 major adaptations
C4 Leaves (separation)
Spatial Separation
Carbon fixation occurs in mesophyll cells
Carbon dioxide is incorporated into 4C organic acid (malate) by the enzyme PEP carboxylase
The 4C acid is then transported to bundle sheath cells, where the carbon dioxide is cleaved from the 4C acid
Since the bundle sheath cells are surrounded by mesophyll, their oxygen gas concentration remains low, even as the light reaction occurs in mesophyll cells
CAM Plants
Carbon fixation occurs during the evening when open stomates will not lead to desiccation
The carbon dioxide is stored in an organic acid
During the day , the organic acid store is used to supply the calvin cycle with carbon dioxide
Importance
Well…
Consider everyone you know, ever pet you have ever had, every ancestor in your lineage…they have all been able to exist for the simple fact that photoautotrophs make more food than they need and produce oxygen gas as a waste product
Also…
Modern industry is more and more interested in using plants to do all sorts of things (like make biofuels, for instance)
 Knowt
Knowt