Cancer Pathophysiology
Cancer
Learning Outcomes:
Define cancer and cancer terminology
Identify incidence and mortality rates
Review cell cycle and examine this process as it relates to carcinogenesis
Compare benign and malignant tumors including cancer cell characteristics
Gain a better understanding of etiology and risk factors for cancer
Describe nomenclature as it relates to neoplasms
Describe classification and staging of malignant tumors
Gain a better understanding of cancer treatment, including side effects and management
Definition
Cancer is the uncontrolled growth of abnormal cells in the body
Cancerous cells are made of less well-differentiated cells that lost ability to control cell proliferation and differentiation into a mature cell
Statistics
Nearly 1 in 2 Canadians will develop cancer at some point in their lives
1 in 4 Canadians will die from cancer at some point in their lives
Cancers of the lung, breast, colon, and prostate account for half of all new cancer cases
Breast cancer more common for women and prostate cancer more common for men
Lung cancer is leading cause of cancer death
Cell cycle
5 phases of the cell cycle
G zero
G1
S (synthesis)
G2
M (mitosis)
Synthesis - DNA is synthesized and chromosomes are replicated
Mitosis - cell divides and 2 daughter cells are formed
G phases- cell is metabolically active or growing enzymes/proteins to prepare for DNA synthesis or mitotic division
After mitosis- daughter cells either go into state of dormancy (G zero phase) where they are not actively proliferating OR if a stimulus for cell division exists, cell enter G1 to begin cell reproductive cycle again
G1 determines overall length of cell cycle because cell spends hours or days in this phase
Differentiation: the process by which proliferating cells become specialized
Categories of differentiation and proliferation cells: cells that never/rarely divide, cells that continue to proliferate then die (PROGENITOR CELLS)
Lastly is stem cells that can enter the cell cycle and produce progenitor cells when required
Cancer cells can complete cell cycle faster by decreasing time spent in G1 phase
Also less likely to enter or remain in G zero phase than normal cells
Cell Cycle Checkpoints
G1-S: monitors whether DNA in chromosomes is damaged by radiation or chemicals
G2-M: prevents entry into mitosis if DNA replication is not complete
Carcinogenesis Intro
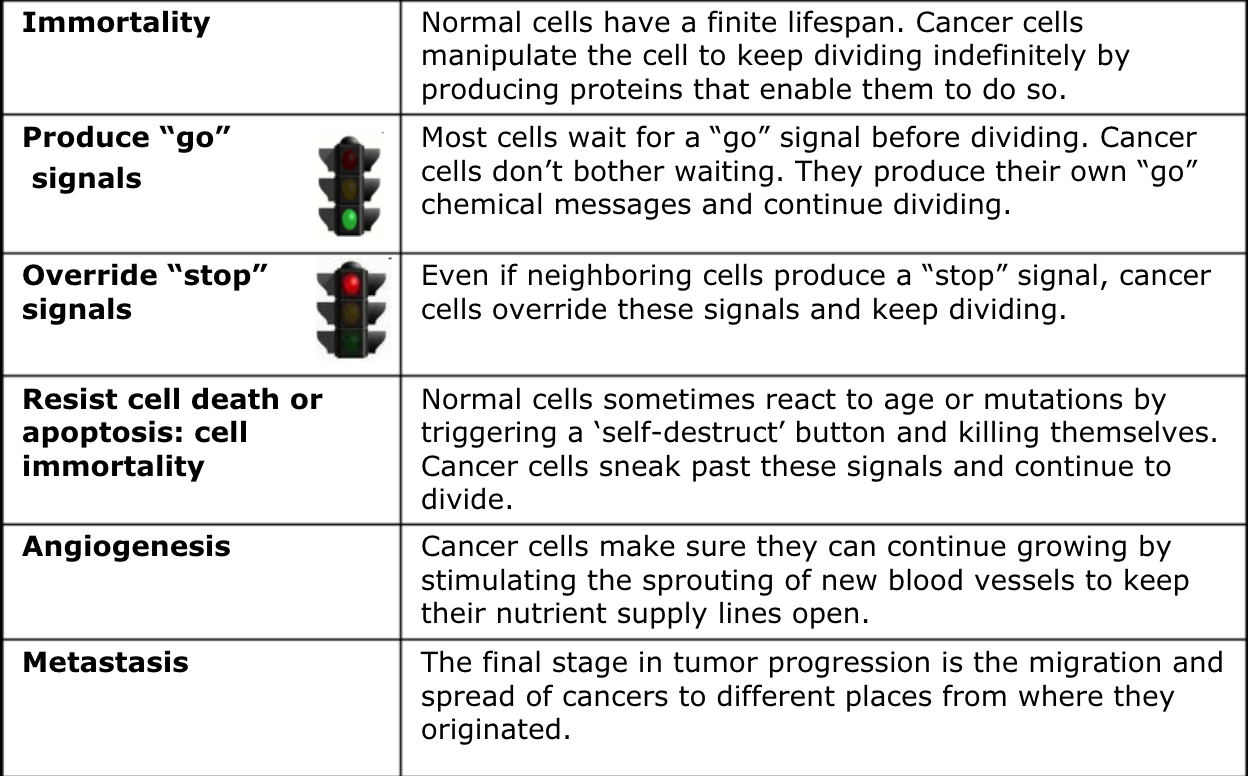
Process by which normal cells are transformed into cancer cells
Caused by mutation of the genetic material of normal cells- upsets normal balance between proliferation and cell death
Results in uncontrolled cell division and tumor development in body
Carcinogenesis Stages
Initiation
Exposure of cells to appropriate doses of carcinogenic agent that makes them susceptible to malignant transformation
Promotion
Unregulated and accelerated growth of the mutated cells and dysplasia
Dysplasia usually indicates early neoplastic process
Progression
Where tumor cells acquire malignant changes and autonomous growth tendencies that promote invasiveness and metastatic capabilities
Carcinoma in situ
Transformation of a neoplastic lesion to one in which cells undergo no maturation and can be considered “cancer like”
Remains localized and hasn’t invaded past the basement membrane into the tissues below surface
Invasive cancer
Cancer that has invaded beyond basement membrane and has potential to metastasize or spread to other body parts
Carcinogenesis- How it occurs?
Proto-oncogenes
Encourage cell division
When mutated they become oncogenes- stimulate excess division
IMPORTANT* oncogenes result form activated or turning on of proto-oncogenes
How it contributes to cancer:
Develop cancer by instructing cells to make proteins or go signals that stimulate excessive cell growth and division
Causing a cell’s growth-signaling pathway to become hyperactive
Tumor suppressor genes (TSG)
Inhibit cell division
When mutated it inactivated these genes causing inhibition of cell division that normally prevents excessive growth
IMPORTANT* cause cancer when they are inactivated/turned off
How it contributes to cancer:
When TSG does not function properly, cells with DNA damage continue to divide and accumulate more DNA damage that eventually lead a cell to grow and divide uncontrollably
Like having a brake pedal that does not work
***people who inherit increased risk of developing cancer are often born with one defective copy of TSG
Because genes come in pairs (one from each parent), inherited defect in one copy will not lead to cancer because other normal copy is still functional
Defective TSG called APC gene causes familial adenomatous polyposis- condition where people develop thousands of colon polyps sometimes leading to colon cancer
DNA Sequencing
3 systems that help avoid runaway cell division
DNA repair system
Instruct a cell to repair damaged DNA
Mutations in DNA repair system:
A change in single base along the base sequence of a gene (like a typo error)
One or more bases added or deleted
Large segments of DNA molecule repeated, deleted or moved
Mutations can lead to failure in repair
When a mistake occur during DNA replication- repair proteins recruit enzyme EXO1 (exonuclease that chops off the mutant strand)
Apoptosis
When old cells become damaged over time they’re eliminated by apoptosis
Tumor suppressor p35 protein initiates cell suicide
Tumor suppressor gene and p35 protein are most frequently mutated genes in human cancer
NK Cells
Can target tumors and cancer cells and kill them
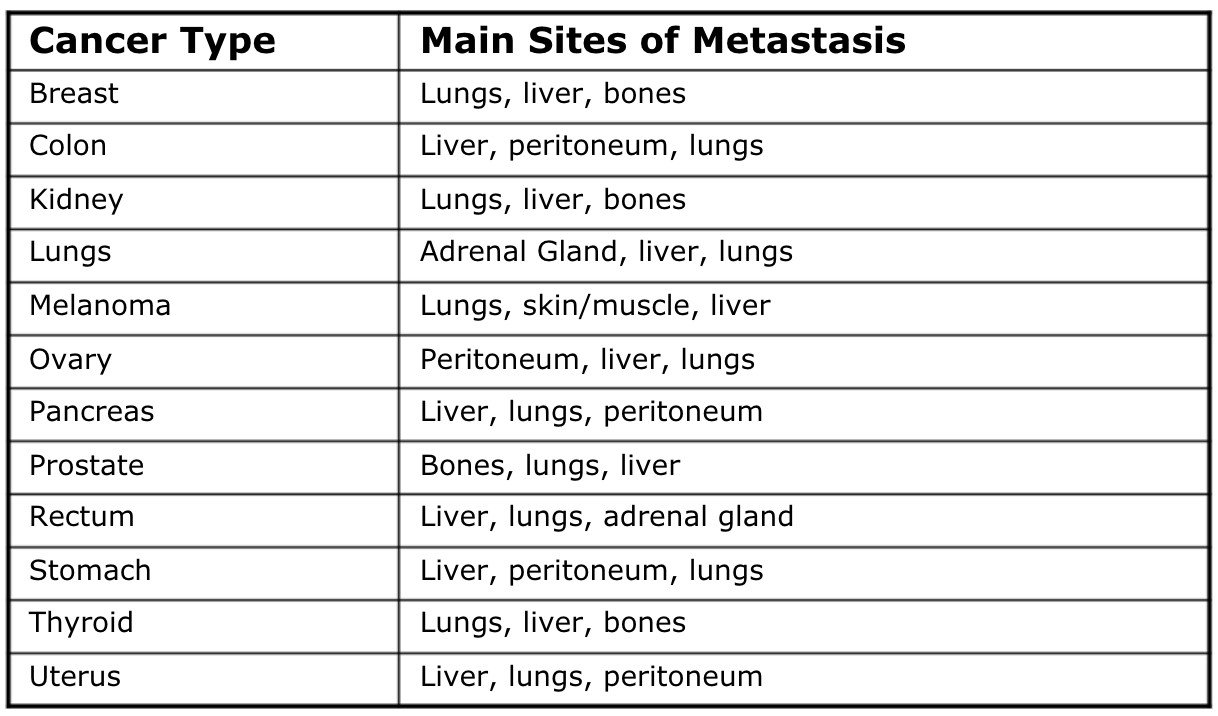
Metastasis
Spread of cancer from original location to other parts of body
Occurs in 2 ways:
Malignant cells directly invade or extend into adjacent organs or sites
Individual cancer cells move away from primary tumor and enter body or lymph circulation
Most common sites are lungs, bones and liver
Angiogenesis
Process of forming new blood vessels
Begins when tumor becomes large enough where it needs to increase supply of nutrients & oxygen
Low oxygen (hypoxia) triggers tumor and environment to release signals that result in growth of BV into the tumor
Tumor Angiogenesis
The proliferation of a network of vessels that penetrates into cancerous growths, supplying nutrients & oxygen and removing waste products
Steps:
Cancerous tumor cells release molecules that send signals to surrounding normal host tissue
Signaling activates certain genes in host tissue that make proteins to encourage growth of new blood vessels
Angiogenesis Inhibitors
Endostatin
Molecules that directly inhibit the growth of endothelial cells
Thalidomide
Prevents endothelial cells from forming new blood vessels
Avastin (first to be FDA approved)
Molecules that interfere with steps in angiogenesis signaling cascade
Delays tumor growth
Interferon-alpha
Naturally occurring protein
Inhibits the production of growth factors from starting the angiogenesis signaling cascade
Etiology
Even though cancer is genetic, only 5-10% if inherited
Chances of getting cancer increase with age
Cancer screening
High risk individuals for prostate cancer should start testing from age 45
High risk: person w/ known gene mutation that increases risk for BC, first degree relative of someone with gene mutation, is assessed as having 25% or greater lifetime risk of breast cancer based on family hx, has had radiation therapy of the chest
Carcinogens: substances directly responsible for damaging DNA, promoting or aiding cancer
Include UV light, radiation, chemical, bacteria, viruses or medical treatments
Lifestyle factors (alcohol, smoking, obesity, inactivity,
Nomenclature
Neoplastic: abnormal growth of new tissue
Benign: noncancerous tumor growth
Composed of well differentiated cells, resembling cells of tissue of origin
Characterized by slow progressive rate of growth that can stop or regress
Lost ability to suppress genetic program for cell proliferation but retained program for cell differentiation
Remain localized to site of origin, lack capacity to infiltrate, invade or metastasize to distant sites
Develop surrounding rim of compressed connective tissue (fibrous capsule)- responsible for sharp line of demarcation between benign tumor and adjacent tissues (known as encapsulated, is a factor for surgical removal)
Named by adding suffix “oma”
Malignant: cancerous tumor growth
Less well differentiated, lost ability to control cell proliferation/differentiation into mature cell
Anaplasia: loss of cell differentiation in cancerous tissue
Poorly differentiated: poorly resembles cell it arose from
Undifferentiated: malignant cells are immature, embryonic and no resemblance to cell it arose from
Grow rapidly in disorganized/uncontrolled manner to invade surrounding tissues and blood vessels
Rob normal tissue of essential nutrients and release enzymes, toxins and cytokines that destroy normal tissue
Have cells that break loose and form metastases
Have suffix “carcinoma” or “sarcoma”
Staging Malignant Tumors
Stage I: small, localized, curable
Stage II: locally advanced
Stage III: locally advanced, lymph node involvement
Stage IV: inoperable, metastatic
IA: no symptoms
IIB: symptoms like fever, night sweats and weight loss
TNM classification: tumor, nodes, metastases
Only lymph nodes draining area of the primary tumor are considered in classification
Molecular Tests
Tumor markers - PSA, CEA, AFP CA125 and Estrogen receptors- occur in blood or tissue useful in patient diagnosis or management
PSA- prostate specific antigen measures levels of PSA in blood, high levels can be marker for prostate cancer
Can also be high in men with infection/inflammation of prostate or benign prostate hyperplasia
CEA- carcinoembryonic antigen
Type of protein that can be found in many different cells of body but usually associated with some tumors
Benign and malignant conditions can increase CEA level
Colon and rectum cancer most commonly increase CEA
***best use of CEA is as a tumor marker- especially for cancers of GI tract
Rising CEA indicates progression or recurrence of cancer
AFP- normal fetal serum protein synthesized by liver, yolk sac and GI
Major component of fetal plasma normally in pregnant women
Rise is usually only seen in diseases like benign liver diseases and hepatocellular carcinoma
CA 125- antigen present on 80% of ovarian carcinomas
Circulates in serum of patients w/ ovarian carcinomas, used as marker to monitor disease
Decrease =good therapy, increase =recurrence
ER+- have receptors for estrogen on surface, growth requires presence of estrogen
ER+ tumors more affected by hormonal treatment and less aggressive
Radiation Therapy
Immediately kills cells, delays or stops cell cycle progression or causes damage to cell’s DNA causing cell death after replication
Chemotherapy
Cells mainly affect by chemo
Blood cell forming bone marrow, hair follicles, lining of the mouth and digestive system
Chemotherapeutic drugs most effective against frequently dividing cells or all phases of cell cycle except G zero
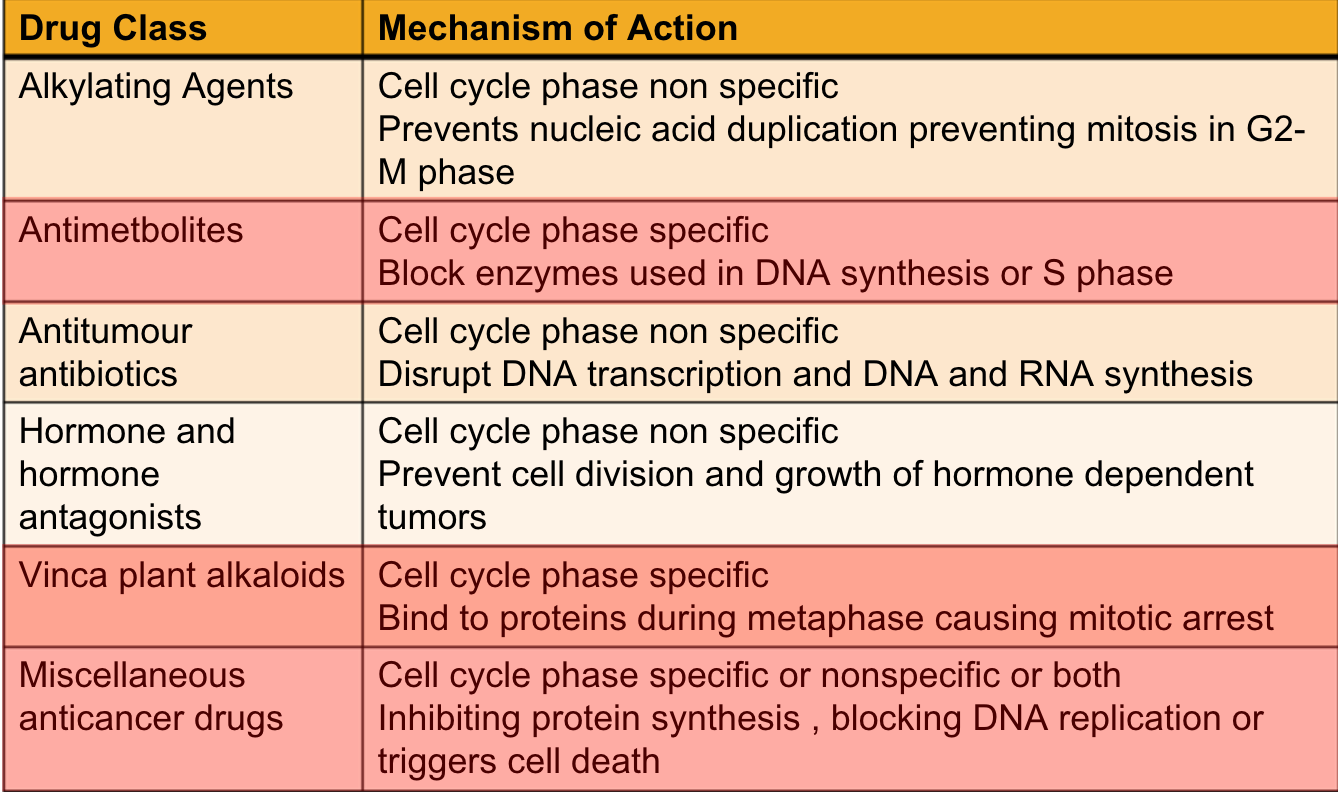
Classifications
Cell cycle phase nonspecific drugs are active on cells in dividing or resting state
Effective on large tumors that have few active cells dividing at time of admin
Usually given as single bolus injections
Cell cycle phase specific drugs are given in minimal concentrations through continuous dosing methods
Hormonal Therapy
Admin of drugs designed to disrupt hormonal environment of cells
Used for cancers that are responsive to or dependent on hormones for growth
Can treat hormone receptors positive breast cancers
By lowering amount of estrogen in body
Or by blocking action of estrogen on breast cancer cells
Estrogen makes hormone receptor positive breast cancers grow
Hormonal therapies are not effective against hormone-receptor-negative breast cancers
Biotherapy
Biologic response modifiers can trigger immune system to indirectly affect tumors
Targeted Therapy
Drugs that selectively attack malignant cells while leaving normal cells unharmed
“Molecularly targeted drugs/therapies”
Interfere with cancer cell division, processes of apoptosis or angiogenesis
Thalidomide
BMT and PBSCT
Restore stem cells that have been destroyed by high doses of chemo or radiation
3 types of transplants
Autologous- patients receive their own stem cells
Syngeneic- patients receive stem cells from identical twin
Allogeneic- patients receive stem cells from brother, sister or parent
Side Effects of Treatment
Intergumentary
Alopecia
Hair loss occurs 10-21 days after drug treatment, is temporary will regrow when drug discontinued
Hair thinning
Local or systemic hypersensitivity reactions
Review pt’s allergy hx, monitor for hypersensitivity of anaphylaxis, test doses as ordered, maintain good hygiene, avoid perfume lotions
Extravasation
Inadvertent leakage of chemo drug from a vessel into surrounding tissue
Assess for immediate/delayed pain, tightness, blister or sloughing of tissues
Prompt admin of antidotes to minimize tissue damage
MSK
Aches/pain
Pain meds
Fatigue
Conserve energy & plan rest periods
Nervous System
Neurotoxicity
Monitor for signs of weakness, numbness, tingling extremities and foot drop
Ototoxicity
Some chemo drugs can cause hearing changes, monitor for tinnitus, hearing loss and vertigo
Sleep pattern disturbances
Vitamins, corticosteroids and neuroleptics for N/V can negatively impact sleep
Anxiety and depression
Set small achievable daily goals, participate in enjoyable and divisional activities and share feelings
Memory changes
“Chemo fog”
Use calendars and lists, provide pill boxes or dosettes
Endocrine System
Hypercalcemia
Monitor serum calcium levels, polyuria and mental status changes
Hyperglycemia
Patients on steroids for cancer can develop high BS
Hyperkalemia
Rapid amount of cellular destruction causes contents of cell to move into blood stream (tumor lysis syndrome)
Hypernatremia
Caused by dehydration, loss of fluids. Monitor serum sodium levels, symptoms of thirst, dry mucous membranes, poor skin turgor, restlessness and lethargy
Hyperuricemia
Monitor serum and urine uric acid levels, daily I/O, rigorous hydration if indicated
Cardiovascular System
Cardiac toxicity
Drugs- cyclophosphamide and doxorubicin
Baseline ECG, echo, cardiac enzymes before chemo, monitor for changes
Digestive System
Hepatotoxicity
Monitor liver function tests, assess for jaundice, tenderness over liver, urine and stool color changes
Anorexia
Eat small frequent meals high in protein, monitor weight
N/V and constipation
Diarrhea
Mucositis/Stomatitis
Symptoms appear 3-5 days after local radiation or systemic chemo
Can be painful enough to require analgesic IV drip
Urinary System
Renal toxicity
Assess baseline, encourage oral intake, monitor I/O and weight changes
Cystitis
Some chemo can cause inflammation and bleeding of bladder lining
Increase fluid intake, empty bladder frequently, administer antidote
Pulmonary System
Pulmonary toxicity
Individuals over 70 at greater risk
Assess baseline resp function, monitor resp status
Reproductive System
Reduced fertility
Fetal death
Lymphatic and Hematological
Neutropenia
Abnormally low count of neutrophils in blood stream (> 2000 cells/cubic mm)
Monitor CBC, infection, sepsis, frequent temperatures, health teaching
Thrombocytopenia
Reduction in number of circulating platelets below 30,000 per cubic mm
Monitor CBC, assess for bruising, purpura, petechiae, nose bleeds, bleeding gums or tarry stools
Platelet transfusions can be required
Anemia
Abnormal or low hematocrit and hemoglobin below 80g/L
Monitor CBC, assess paleness, chest pain, SOB, heart palpitations, dizziness, lethargy
Cancer Pathophysiology
Cancer
Learning Outcomes:
Define cancer and cancer terminology
Identify incidence and mortality rates
Review cell cycle and examine this process as it relates to carcinogenesis
Compare benign and malignant tumors including cancer cell characteristics
Gain a better understanding of etiology and risk factors for cancer
Describe nomenclature as it relates to neoplasms
Describe classification and staging of malignant tumors
Gain a better understanding of cancer treatment, including side effects and management
Definition
Cancer is the uncontrolled growth of abnormal cells in the body
Cancerous cells are made of less well-differentiated cells that lost ability to control cell proliferation and differentiation into a mature cell
Statistics
Nearly 1 in 2 Canadians will develop cancer at some point in their lives
1 in 4 Canadians will die from cancer at some point in their lives
Cancers of the lung, breast, colon, and prostate account for half of all new cancer cases
Breast cancer more common for women and prostate cancer more common for men
Lung cancer is leading cause of cancer death
Cell cycle
5 phases of the cell cycle
G zero
G1
S (synthesis)
G2
M (mitosis)
Synthesis - DNA is synthesized and chromosomes are replicated
Mitosis - cell divides and 2 daughter cells are formed
G phases- cell is metabolically active or growing enzymes/proteins to prepare for DNA synthesis or mitotic division
After mitosis- daughter cells either go into state of dormancy (G zero phase) where they are not actively proliferating OR if a stimulus for cell division exists, cell enter G1 to begin cell reproductive cycle again
G1 determines overall length of cell cycle because cell spends hours or days in this phase
Differentiation: the process by which proliferating cells become specialized
Categories of differentiation and proliferation cells: cells that never/rarely divide, cells that continue to proliferate then die (PROGENITOR CELLS)
Lastly is stem cells that can enter the cell cycle and produce progenitor cells when required
Cancer cells can complete cell cycle faster by decreasing time spent in G1 phase
Also less likely to enter or remain in G zero phase than normal cells
Cell Cycle Checkpoints
G1-S: monitors whether DNA in chromosomes is damaged by radiation or chemicals
G2-M: prevents entry into mitosis if DNA replication is not complete
Carcinogenesis Intro
Process by which normal cells are transformed into cancer cells
Caused by mutation of the genetic material of normal cells- upsets normal balance between proliferation and cell death
Results in uncontrolled cell division and tumor development in body
Carcinogenesis Stages
Initiation
Exposure of cells to appropriate doses of carcinogenic agent that makes them susceptible to malignant transformation
Promotion
Unregulated and accelerated growth of the mutated cells and dysplasia
Dysplasia usually indicates early neoplastic process
Progression
Where tumor cells acquire malignant changes and autonomous growth tendencies that promote invasiveness and metastatic capabilities
Carcinoma in situ
Transformation of a neoplastic lesion to one in which cells undergo no maturation and can be considered “cancer like”
Remains localized and hasn’t invaded past the basement membrane into the tissues below surface
Invasive cancer
Cancer that has invaded beyond basement membrane and has potential to metastasize or spread to other body parts
Carcinogenesis- How it occurs?
Proto-oncogenes
Encourage cell division
When mutated they become oncogenes- stimulate excess division
IMPORTANT* oncogenes result form activated or turning on of proto-oncogenes
How it contributes to cancer:
Develop cancer by instructing cells to make proteins or go signals that stimulate excessive cell growth and division
Causing a cell’s growth-signaling pathway to become hyperactive
Tumor suppressor genes (TSG)
Inhibit cell division
When mutated it inactivated these genes causing inhibition of cell division that normally prevents excessive growth
IMPORTANT* cause cancer when they are inactivated/turned off
How it contributes to cancer:
When TSG does not function properly, cells with DNA damage continue to divide and accumulate more DNA damage that eventually lead a cell to grow and divide uncontrollably
Like having a brake pedal that does not work
***people who inherit increased risk of developing cancer are often born with one defective copy of TSG
Because genes come in pairs (one from each parent), inherited defect in one copy will not lead to cancer because other normal copy is still functional
Defective TSG called APC gene causes familial adenomatous polyposis- condition where people develop thousands of colon polyps sometimes leading to colon cancer
DNA Sequencing
3 systems that help avoid runaway cell division
DNA repair system
Instruct a cell to repair damaged DNA
Mutations in DNA repair system:
A change in single base along the base sequence of a gene (like a typo error)
One or more bases added or deleted
Large segments of DNA molecule repeated, deleted or moved
Mutations can lead to failure in repair
When a mistake occur during DNA replication- repair proteins recruit enzyme EXO1 (exonuclease that chops off the mutant strand)
Apoptosis
When old cells become damaged over time they’re eliminated by apoptosis
Tumor suppressor p35 protein initiates cell suicide
Tumor suppressor gene and p35 protein are most frequently mutated genes in human cancer
NK Cells
Can target tumors and cancer cells and kill them
Metastasis
Spread of cancer from original location to other parts of body
Occurs in 2 ways:
Malignant cells directly invade or extend into adjacent organs or sites
Individual cancer cells move away from primary tumor and enter body or lymph circulation
Most common sites are lungs, bones and liver
Angiogenesis
Process of forming new blood vessels
Begins when tumor becomes large enough where it needs to increase supply of nutrients & oxygen
Low oxygen (hypoxia) triggers tumor and environment to release signals that result in growth of BV into the tumor
Tumor Angiogenesis
The proliferation of a network of vessels that penetrates into cancerous growths, supplying nutrients & oxygen and removing waste products
Steps:
Cancerous tumor cells release molecules that send signals to surrounding normal host tissue
Signaling activates certain genes in host tissue that make proteins to encourage growth of new blood vessels
Angiogenesis Inhibitors
Endostatin
Molecules that directly inhibit the growth of endothelial cells
Thalidomide
Prevents endothelial cells from forming new blood vessels
Avastin (first to be FDA approved)
Molecules that interfere with steps in angiogenesis signaling cascade
Delays tumor growth
Interferon-alpha
Naturally occurring protein
Inhibits the production of growth factors from starting the angiogenesis signaling cascade
Etiology
Even though cancer is genetic, only 5-10% if inherited
Chances of getting cancer increase with age
Cancer screening
High risk individuals for prostate cancer should start testing from age 45
High risk: person w/ known gene mutation that increases risk for BC, first degree relative of someone with gene mutation, is assessed as having 25% or greater lifetime risk of breast cancer based on family hx, has had radiation therapy of the chest
Carcinogens: substances directly responsible for damaging DNA, promoting or aiding cancer
Include UV light, radiation, chemical, bacteria, viruses or medical treatments
Lifestyle factors (alcohol, smoking, obesity, inactivity,
Nomenclature
Neoplastic: abnormal growth of new tissue
Benign: noncancerous tumor growth
Composed of well differentiated cells, resembling cells of tissue of origin
Characterized by slow progressive rate of growth that can stop or regress
Lost ability to suppress genetic program for cell proliferation but retained program for cell differentiation
Remain localized to site of origin, lack capacity to infiltrate, invade or metastasize to distant sites
Develop surrounding rim of compressed connective tissue (fibrous capsule)- responsible for sharp line of demarcation between benign tumor and adjacent tissues (known as encapsulated, is a factor for surgical removal)
Named by adding suffix “oma”
Malignant: cancerous tumor growth
Less well differentiated, lost ability to control cell proliferation/differentiation into mature cell
Anaplasia: loss of cell differentiation in cancerous tissue
Poorly differentiated: poorly resembles cell it arose from
Undifferentiated: malignant cells are immature, embryonic and no resemblance to cell it arose from
Grow rapidly in disorganized/uncontrolled manner to invade surrounding tissues and blood vessels
Rob normal tissue of essential nutrients and release enzymes, toxins and cytokines that destroy normal tissue
Have cells that break loose and form metastases
Have suffix “carcinoma” or “sarcoma”
Staging Malignant Tumors
Stage I: small, localized, curable
Stage II: locally advanced
Stage III: locally advanced, lymph node involvement
Stage IV: inoperable, metastatic
IA: no symptoms
IIB: symptoms like fever, night sweats and weight loss
TNM classification: tumor, nodes, metastases
Only lymph nodes draining area of the primary tumor are considered in classification
Molecular Tests
Tumor markers - PSA, CEA, AFP CA125 and Estrogen receptors- occur in blood or tissue useful in patient diagnosis or management
PSA- prostate specific antigen measures levels of PSA in blood, high levels can be marker for prostate cancer
Can also be high in men with infection/inflammation of prostate or benign prostate hyperplasia
CEA- carcinoembryonic antigen
Type of protein that can be found in many different cells of body but usually associated with some tumors
Benign and malignant conditions can increase CEA level
Colon and rectum cancer most commonly increase CEA
***best use of CEA is as a tumor marker- especially for cancers of GI tract
Rising CEA indicates progression or recurrence of cancer
AFP- normal fetal serum protein synthesized by liver, yolk sac and GI
Major component of fetal plasma normally in pregnant women
Rise is usually only seen in diseases like benign liver diseases and hepatocellular carcinoma
CA 125- antigen present on 80% of ovarian carcinomas
Circulates in serum of patients w/ ovarian carcinomas, used as marker to monitor disease
Decrease =good therapy, increase =recurrence
ER+- have receptors for estrogen on surface, growth requires presence of estrogen
ER+ tumors more affected by hormonal treatment and less aggressive
Radiation Therapy
Immediately kills cells, delays or stops cell cycle progression or causes damage to cell’s DNA causing cell death after replication
Chemotherapy
Cells mainly affect by chemo
Blood cell forming bone marrow, hair follicles, lining of the mouth and digestive system
Chemotherapeutic drugs most effective against frequently dividing cells or all phases of cell cycle except G zero
Classifications
Cell cycle phase nonspecific drugs are active on cells in dividing or resting state
Effective on large tumors that have few active cells dividing at time of admin
Usually given as single bolus injections
Cell cycle phase specific drugs are given in minimal concentrations through continuous dosing methods
Hormonal Therapy
Admin of drugs designed to disrupt hormonal environment of cells
Used for cancers that are responsive to or dependent on hormones for growth
Can treat hormone receptors positive breast cancers
By lowering amount of estrogen in body
Or by blocking action of estrogen on breast cancer cells
Estrogen makes hormone receptor positive breast cancers grow
Hormonal therapies are not effective against hormone-receptor-negative breast cancers
Biotherapy
Biologic response modifiers can trigger immune system to indirectly affect tumors
Targeted Therapy
Drugs that selectively attack malignant cells while leaving normal cells unharmed
“Molecularly targeted drugs/therapies”
Interfere with cancer cell division, processes of apoptosis or angiogenesis
Thalidomide
BMT and PBSCT
Restore stem cells that have been destroyed by high doses of chemo or radiation
3 types of transplants
Autologous- patients receive their own stem cells
Syngeneic- patients receive stem cells from identical twin
Allogeneic- patients receive stem cells from brother, sister or parent
Side Effects of Treatment
Intergumentary
Alopecia
Hair loss occurs 10-21 days after drug treatment, is temporary will regrow when drug discontinued
Hair thinning
Local or systemic hypersensitivity reactions
Review pt’s allergy hx, monitor for hypersensitivity of anaphylaxis, test doses as ordered, maintain good hygiene, avoid perfume lotions
Extravasation
Inadvertent leakage of chemo drug from a vessel into surrounding tissue
Assess for immediate/delayed pain, tightness, blister or sloughing of tissues
Prompt admin of antidotes to minimize tissue damage
MSK
Aches/pain
Pain meds
Fatigue
Conserve energy & plan rest periods
Nervous System
Neurotoxicity
Monitor for signs of weakness, numbness, tingling extremities and foot drop
Ototoxicity
Some chemo drugs can cause hearing changes, monitor for tinnitus, hearing loss and vertigo
Sleep pattern disturbances
Vitamins, corticosteroids and neuroleptics for N/V can negatively impact sleep
Anxiety and depression
Set small achievable daily goals, participate in enjoyable and divisional activities and share feelings
Memory changes
“Chemo fog”
Use calendars and lists, provide pill boxes or dosettes
Endocrine System
Hypercalcemia
Monitor serum calcium levels, polyuria and mental status changes
Hyperglycemia
Patients on steroids for cancer can develop high BS
Hyperkalemia
Rapid amount of cellular destruction causes contents of cell to move into blood stream (tumor lysis syndrome)
Hypernatremia
Caused by dehydration, loss of fluids. Monitor serum sodium levels, symptoms of thirst, dry mucous membranes, poor skin turgor, restlessness and lethargy
Hyperuricemia
Monitor serum and urine uric acid levels, daily I/O, rigorous hydration if indicated
Cardiovascular System
Cardiac toxicity
Drugs- cyclophosphamide and doxorubicin
Baseline ECG, echo, cardiac enzymes before chemo, monitor for changes
Digestive System
Hepatotoxicity
Monitor liver function tests, assess for jaundice, tenderness over liver, urine and stool color changes
Anorexia
Eat small frequent meals high in protein, monitor weight
N/V and constipation
Diarrhea
Mucositis/Stomatitis
Symptoms appear 3-5 days after local radiation or systemic chemo
Can be painful enough to require analgesic IV drip
Urinary System
Renal toxicity
Assess baseline, encourage oral intake, monitor I/O and weight changes
Cystitis
Some chemo can cause inflammation and bleeding of bladder lining
Increase fluid intake, empty bladder frequently, administer antidote
Pulmonary System
Pulmonary toxicity
Individuals over 70 at greater risk
Assess baseline resp function, monitor resp status
Reproductive System
Reduced fertility
Fetal death
Lymphatic and Hematological
Neutropenia
Abnormally low count of neutrophils in blood stream (> 2000 cells/cubic mm)
Monitor CBC, infection, sepsis, frequent temperatures, health teaching
Thrombocytopenia
Reduction in number of circulating platelets below 30,000 per cubic mm
Monitor CBC, assess for bruising, purpura, petechiae, nose bleeds, bleeding gums or tarry stools
Platelet transfusions can be required
Anemia
Abnormal or low hematocrit and hemoglobin below 80g/L
Monitor CBC, assess paleness, chest pain, SOB, heart palpitations, dizziness, lethargy
 Knowt
Knowt