
Chapter 19- Ammonia
Reactions going either way (forwards and backwards) are called reversible reactions. A double-headed arrow is used to represent reversible reactions.
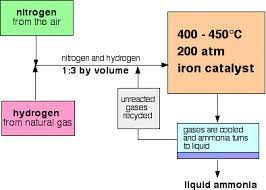
The Haber process (the process of manufacturing ammonia) is a reversible reaction.
Hydrogen and nitrogen are the raw materials. Nitrogen is extracted from the air by fractional distillation and hydrogen is obtained by the cracking of hydrocarbons.
Since nitrogen is unreactive, a high temperature of 450 degrees celsius is required for the haber process.’
Iron is used as a catalyst to speed up the reaction.
CONDITIONS FOR THE HABER PROCESS
To ensure maximum yield of ammonia:
a lower temperature is used (although it reduces the rate of reaction)
high pressure is used (although it increases the cost for the process)
iron is used a catalyst (it speeds up the reaction but doesn’t affect the amount of yield. )
DISPLACEMENT OF AMMONIUM SALTS
When an ammonium salt is heated with an alkali, ammonia gas is produced.
Chapter 19- Ammonia
Reactions going either way (forwards and backwards) are called reversible reactions. A double-headed arrow is used to represent reversible reactions.
The Haber process (the process of manufacturing ammonia) is a reversible reaction.
Hydrogen and nitrogen are the raw materials. Nitrogen is extracted from the air by fractional distillation and hydrogen is obtained by the cracking of hydrocarbons.
Since nitrogen is unreactive, a high temperature of 450 degrees celsius is required for the haber process.’
Iron is used as a catalyst to speed up the reaction.
CONDITIONS FOR THE HABER PROCESS
To ensure maximum yield of ammonia:
a lower temperature is used (although it reduces the rate of reaction)
high pressure is used (although it increases the cost for the process)
iron is used a catalyst (it speeds up the reaction but doesn’t affect the amount of yield. )
DISPLACEMENT OF AMMONIUM SALTS
When an ammonium salt is heated with an alkali, ammonia gas is produced.

 Knowt
Knowt
