
AP BIO Unit 3 Review - Cellular Energetics
Enzymes and Reactions
Entropy -A measure of disorder, or randomness
decreased by nonspontaneous reactions
increase by heat/energy released
Energy Coupling - In cellular metabolism, the use of energy released from an exergonic reaction to drive an endergonic reaction
Photosynthesis transforms light energy into chemical energy stored in glucose, and cellular respiration releases the energy from glucose to build ATP, which does the work of life
Active Site - The specific region of an enzyme that binds the substrate and that forms the pocket in which catalysis occurs
Because systems at equilibrium are at a minimum of G and can do no work, a cell that has reached metabolic equilibrium is dead! The fact that metabolism as a whole is never at equilibrium is one of the defining features of life
Types of reactions
Catabolic/Exergonic/Spontaneous
doesn’t require the input of energy

Anabolic/Endergonic/Nonspontaneous
requires the input of energy
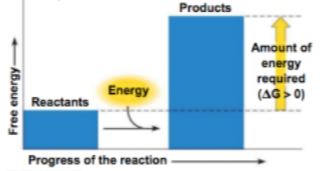
Enzymes lower the activation energy required for a reaction
specific for the reactions they catalyze
orients substrates properly
stretch and stress bonds to be broken
provides an ideal environment (pH, temperature)
Properties of Enzymes
Environment conditions

Shape changes
Inhibition
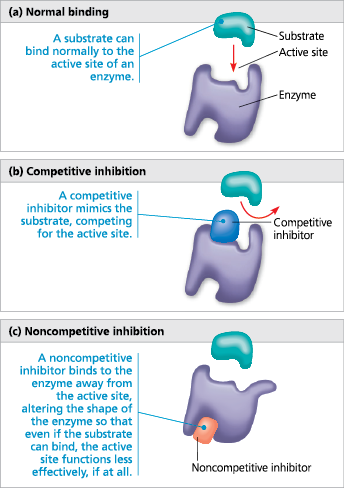
Oscillation

Feedback
End product allosterically inhibit - prevents the cell from creating more product than necessary
ATP binds to several catabolic enzymes allosterically, lowering their affinity for substrate and thus inhibiting their activity. ADP, however, functions as an activator of the same enzymes. This is logical because catabolism functions in regenerating ATP. If ATP production lags behind its use, ADP accumulates and activates the enzymes that speed up catabolism, producing more ATP. If the supply of ATP exceeds demand, then catabolism slows down as ATP molecules accumulate and bind to the same enzymes, inhibiting them

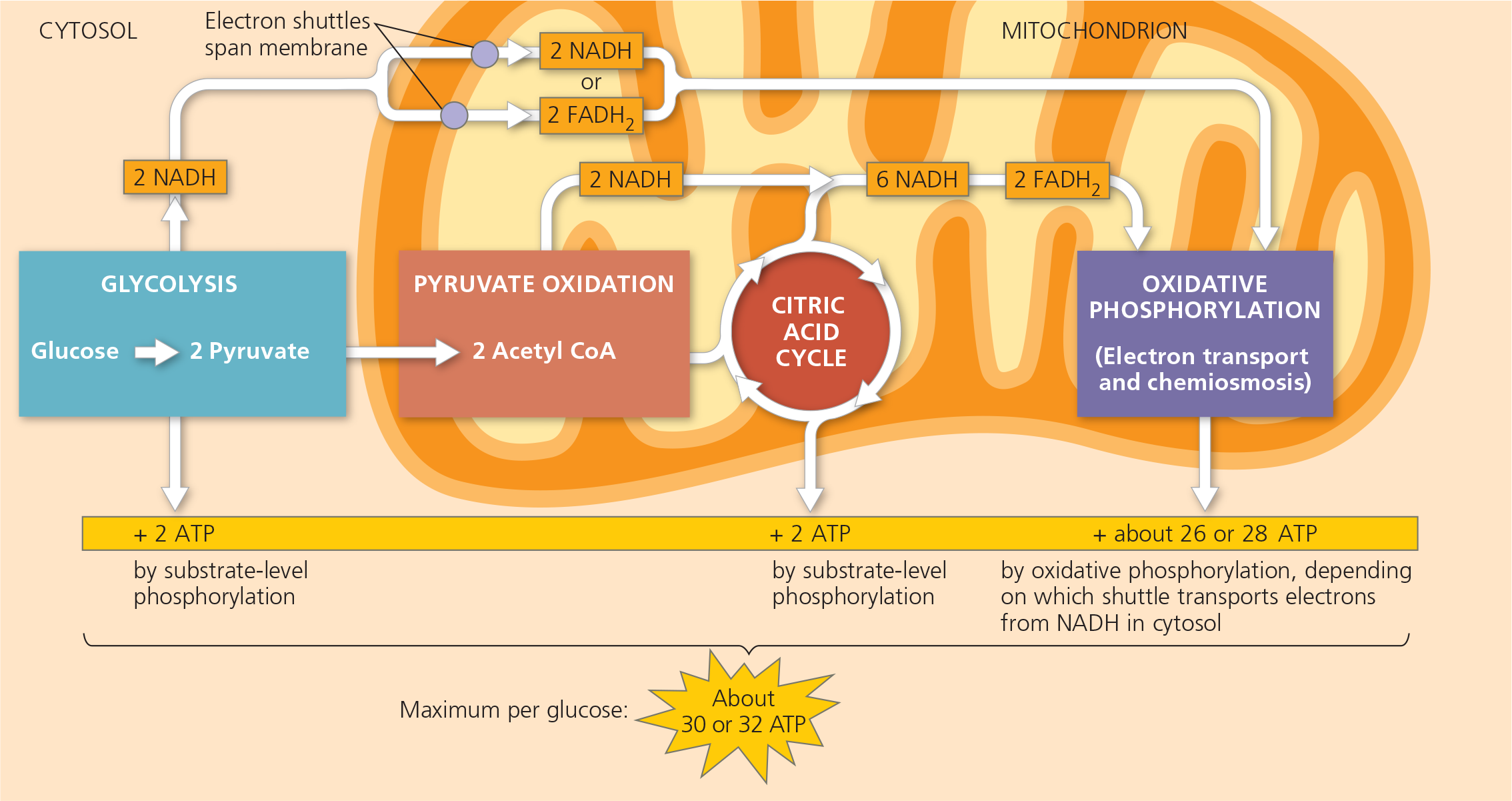
Cellular Respiration
C6H12O6 + 6O2 → 6CO2 + 6H2O + Energy
Glycolysis
A series of reactions that ultimately splits glucose into pyruvate. Glycolysis occurs in almost all living cells, serving as the starting point for fermentation or cellular respiration
occurs in the cytoplasm
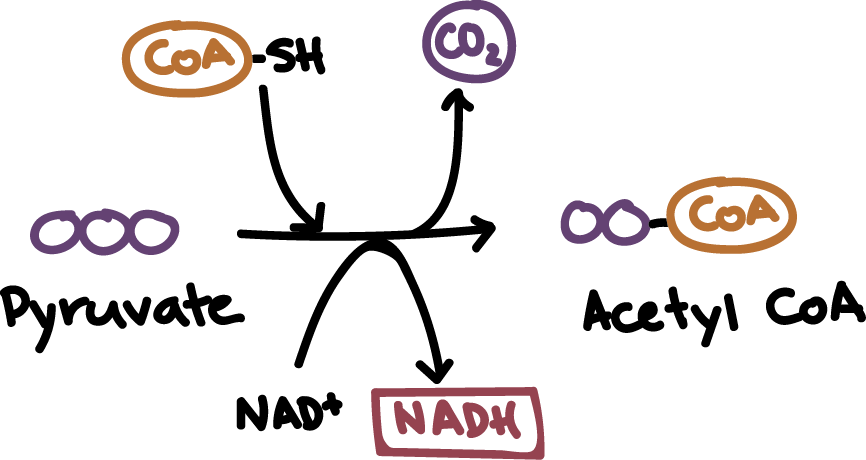
Pyruvate Oxidation
A biochemical reaction that involves the oxidation of pyruvate to create acetyl CoA
occurs in the mitochondria
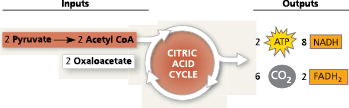
Citric Acid Cycle
A chemical cycle involving eight steps that completes the metabolic breakdown of glucose molecules begun in glycolysis by oxidizing acetyl CoA (derived from pyruvate) to carbon dioxide; occurs within the mitochondrion in eukaryotic cells and in the cytosol of prokaryotes; together with pyruvate oxidation, the second major stage in cellular respiration
occurs in the mitochondria
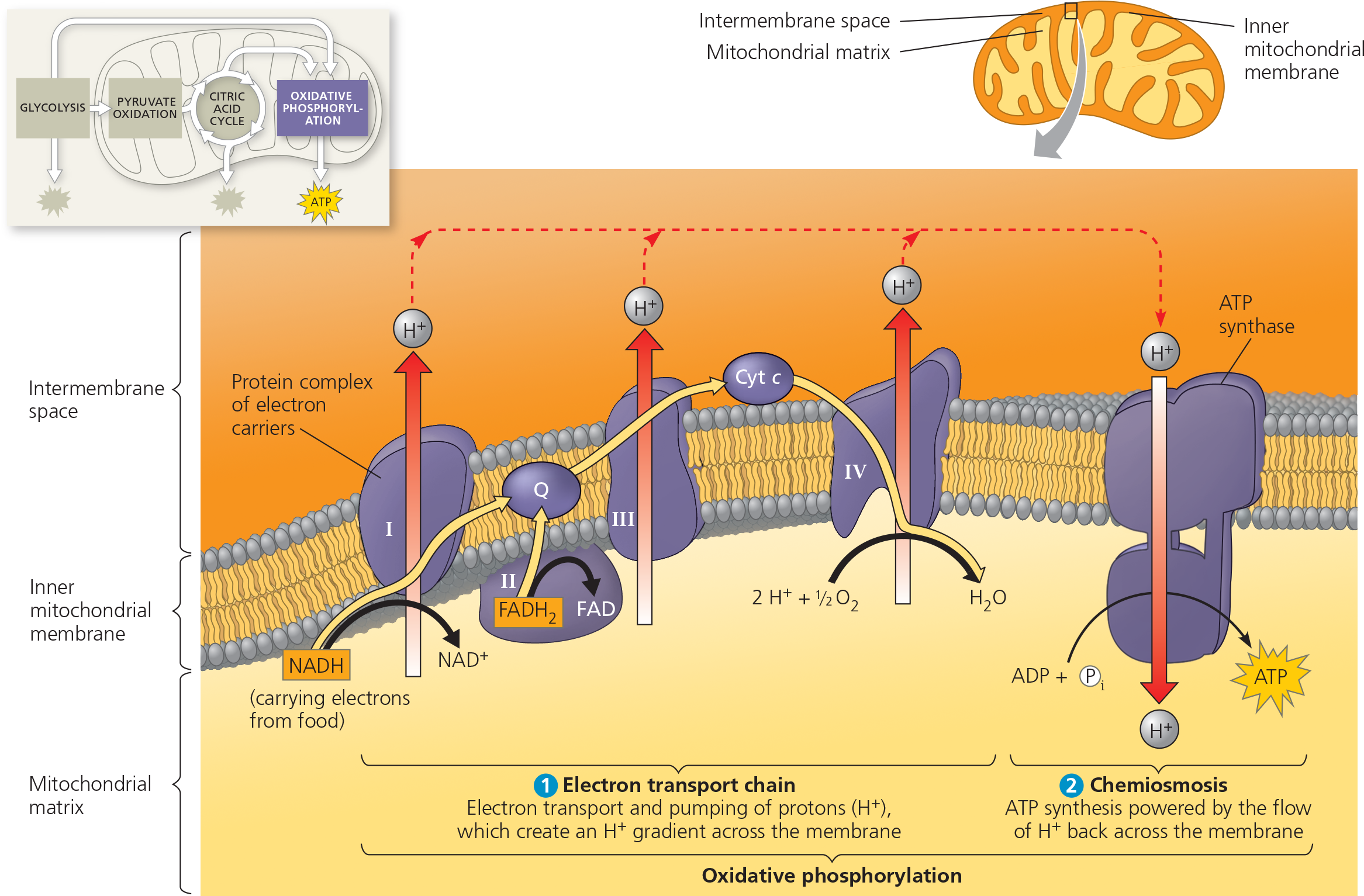
Oxidative Phosphorylation
The production of ATP using energy derived from the redox reactions of an electron transport chain; the third major stage of cellular respiration
occurs in the mitochondria
ETC in the inner membrane (NADH and FADH2 drops of elections and H+ goes to the intermembrane space through proteins)
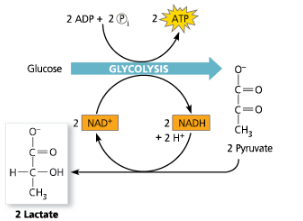
Fermentation
A catabolic process that makes a limited amount of ATP from glucose (or other organic molecules) without an electron transport chain and that produces a characteristic end product, such as ethyl alcohol or lactic acid

Importantce of Oxygen
O2 is the final electron acceptor
Anaerobic -A catabolic pathway in which inorganic molecules other than oxygen accept electrons at the “downhill” end of electron transport chains
Aerobic - A catabolic pathway for organic molecules, using oxygen (O2) as the final electron acceptor in an electron transport chain and ultimately producing ATP. This is the most efficient catabolic pathway and is carried out in most eukaryotic cells and many prokaryotic organisms
Alcohol Fermentation - Glycolysis followed by the reduction of pyruvate to ethyl alcohol, regenerating NAD+ and releasing carbon dioxide

Lactic Acid Fermentation - Glycolysis followed by the reduction of pyruvate to lactate, regenerating NAD+ with no release of carbon dioxide
Photosynthesis
6CO2 + 12H2O + Light Energy → C6H12O6 + 6O2

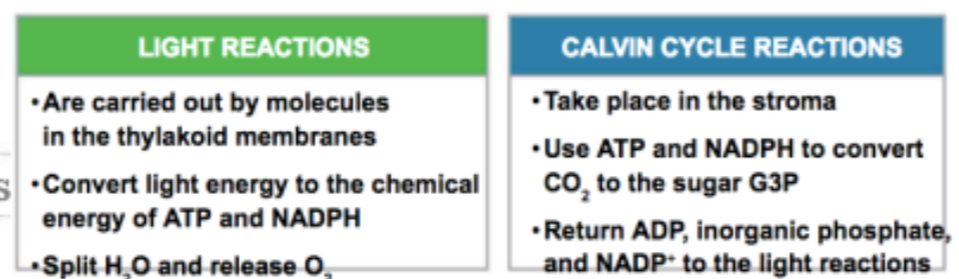
Light Reactions
The first of two major stages in photosynthesis (preceding the Calvin cycle). These reactions, which occur on the thylakoid membranes of the chloroplast or on membranes of certain prokaryotes, convert solar energy to the chemical energy of ATP and NADPH, releasing oxygen in the process
energy harvesting phase
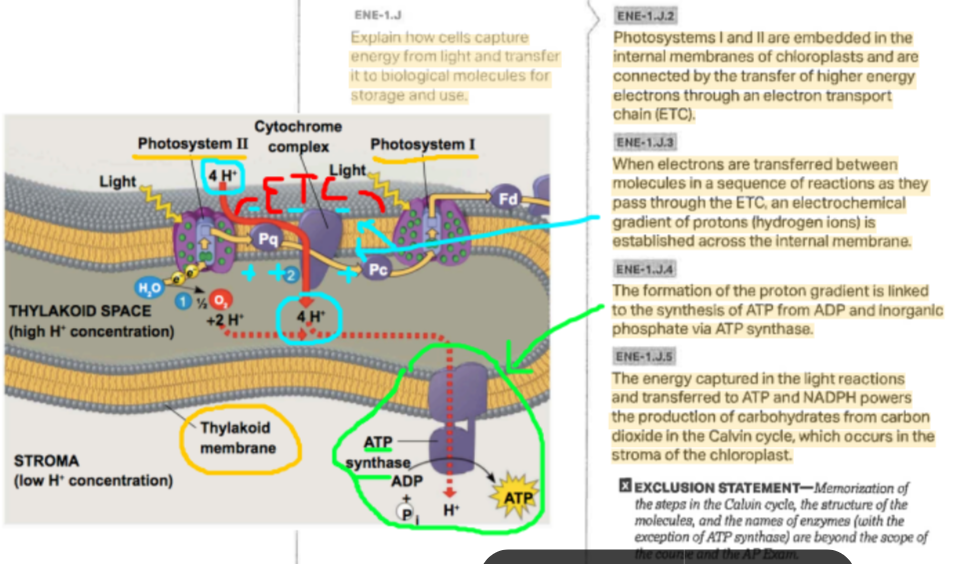
Photophosphorylation
The process of generating ATP from ADP and phosphate by means of chemiosmosis, using a proton-motive force generated across the thylakoid membrane of the chloroplast or the membrane of certain prokaryotes during the light reactions of photosynthesis
Calvin Cycle
The second of two major stages in photosynthesis (following the light reactions), involving fixation of atmospheric CO2 and reduction of the fixed carbon into carbohydrate
forms sugar
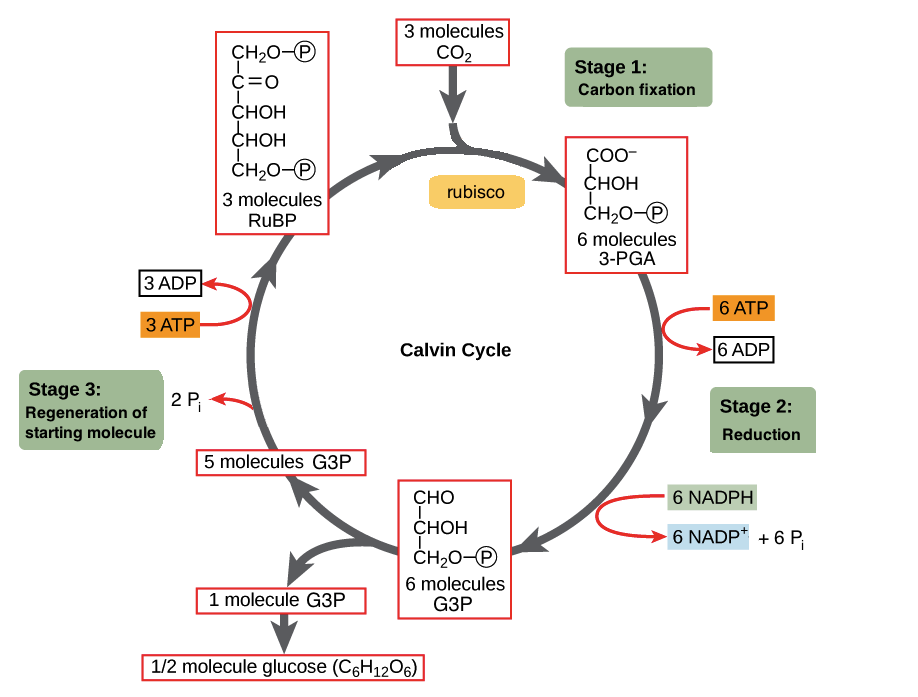
Carbon Fixation
The initial incorporation of carbon from CO2 into an organic compound by an autotrophic organism (a plant, another photosynthetic organism, or a chemoautotrophic prokaryote)
AP BIO Unit 3 Review - Cellular Energetics
Enzymes and Reactions
Entropy -A measure of disorder, or randomness
decreased by nonspontaneous reactions
increase by heat/energy released
Energy Coupling - In cellular metabolism, the use of energy released from an exergonic reaction to drive an endergonic reaction
Photosynthesis transforms light energy into chemical energy stored in glucose, and cellular respiration releases the energy from glucose to build ATP, which does the work of life
Active Site - The specific region of an enzyme that binds the substrate and that forms the pocket in which catalysis occurs
Because systems at equilibrium are at a minimum of G and can do no work, a cell that has reached metabolic equilibrium is dead! The fact that metabolism as a whole is never at equilibrium is one of the defining features of life
Types of reactions
Catabolic/Exergonic/Spontaneous
doesn’t require the input of energy

Anabolic/Endergonic/Nonspontaneous
requires the input of energy

Enzymes lower the activation energy required for a reaction
specific for the reactions they catalyze
orients substrates properly
stretch and stress bonds to be broken
provides an ideal environment (pH, temperature)
Properties of Enzymes
Environment conditions

Shape changes
Inhibition

Oscillation

Feedback
End product allosterically inhibit - prevents the cell from creating more product than necessary
ATP binds to several catabolic enzymes allosterically, lowering their affinity for substrate and thus inhibiting their activity. ADP, however, functions as an activator of the same enzymes. This is logical because catabolism functions in regenerating ATP. If ATP production lags behind its use, ADP accumulates and activates the enzymes that speed up catabolism, producing more ATP. If the supply of ATP exceeds demand, then catabolism slows down as ATP molecules accumulate and bind to the same enzymes, inhibiting them

Cellular Respiration
C6H12O6 + 6O2 → 6CO2 + 6H2O + Energy
Glycolysis
A series of reactions that ultimately splits glucose into pyruvate. Glycolysis occurs in almost all living cells, serving as the starting point for fermentation or cellular respiration
occurs in the cytoplasm
Pyruvate Oxidation
A biochemical reaction that involves the oxidation of pyruvate to create acetyl CoA
occurs in the mitochondria
Citric Acid Cycle
A chemical cycle involving eight steps that completes the metabolic breakdown of glucose molecules begun in glycolysis by oxidizing acetyl CoA (derived from pyruvate) to carbon dioxide; occurs within the mitochondrion in eukaryotic cells and in the cytosol of prokaryotes; together with pyruvate oxidation, the second major stage in cellular respiration
occurs in the mitochondria
Oxidative Phosphorylation
The production of ATP using energy derived from the redox reactions of an electron transport chain; the third major stage of cellular respiration
occurs in the mitochondria
ETC in the inner membrane (NADH and FADH2 drops of elections and H+ goes to the intermembrane space through proteins)
Fermentation
A catabolic process that makes a limited amount of ATP from glucose (or other organic molecules) without an electron transport chain and that produces a characteristic end product, such as ethyl alcohol or lactic acid





Importantce of Oxygen
O2 is the final electron acceptor
Anaerobic -A catabolic pathway in which inorganic molecules other than oxygen accept electrons at the “downhill” end of electron transport chains
Aerobic - A catabolic pathway for organic molecules, using oxygen (O2) as the final electron acceptor in an electron transport chain and ultimately producing ATP. This is the most efficient catabolic pathway and is carried out in most eukaryotic cells and many prokaryotic organisms
Alcohol Fermentation - Glycolysis followed by the reduction of pyruvate to ethyl alcohol, regenerating NAD+ and releasing carbon dioxide

Lactic Acid Fermentation - Glycolysis followed by the reduction of pyruvate to lactate, regenerating NAD+ with no release of carbon dioxide

Photosynthesis
6CO2 + 12H2O + Light Energy → C6H12O6 + 6O2


Light Reactions
The first of two major stages in photosynthesis (preceding the Calvin cycle). These reactions, which occur on the thylakoid membranes of the chloroplast or on membranes of certain prokaryotes, convert solar energy to the chemical energy of ATP and NADPH, releasing oxygen in the process
energy harvesting phase

Photophosphorylation
The process of generating ATP from ADP and phosphate by means of chemiosmosis, using a proton-motive force generated across the thylakoid membrane of the chloroplast or the membrane of certain prokaryotes during the light reactions of photosynthesis
Calvin Cycle
The second of two major stages in photosynthesis (following the light reactions), involving fixation of atmospheric CO2 and reduction of the fixed carbon into carbohydrate
forms sugar

Carbon Fixation
The initial incorporation of carbon from CO2 into an organic compound by an autotrophic organism (a plant, another photosynthetic organism, or a chemoautotrophic prokaryote)
 Knowt
Knowt
